Tarnish on silver is the compound Ag2O. A tarnished silver spoon is placed in an aluminum pan of boiling water. When enough salt is added so that the solution conducts electricity, the tarnish disappears. Imagine that the two halves of this redox reaction were separated and connected with a wire and a salt bridge. Part A Calculate the standard cell potential given the following standard reduction potentials: Al+ +3e Al; E =-1.66 V Agt +eAg; E =0.799 V Express your answer to two decimal places and include the appropriate units. > View Available Hint(s) Eo cell Value Units
Tarnish on silver is the compound Ag2O. A tarnished silver spoon is placed in an aluminum pan of boiling water. When enough salt is added so that the solution conducts electricity, the tarnish disappears. Imagine that the two halves of this redox reaction were separated and connected with a wire and a salt bridge. Part A Calculate the standard cell potential given the following standard reduction potentials: Al+ +3e Al; E =-1.66 V Agt +eAg; E =0.799 V Express your answer to two decimal places and include the appropriate units. > View Available Hint(s) Eo cell Value Units
Chemistry: An Atoms First Approach
2nd Edition
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Steven S. Zumdahl, Susan A. Zumdahl
Chapter17: Electrochemistry
Section: Chapter Questions
Problem 135CWP: Consider a galvanic cell based on the following half-reactions: a. What is the expected cell...
Related questions
Question
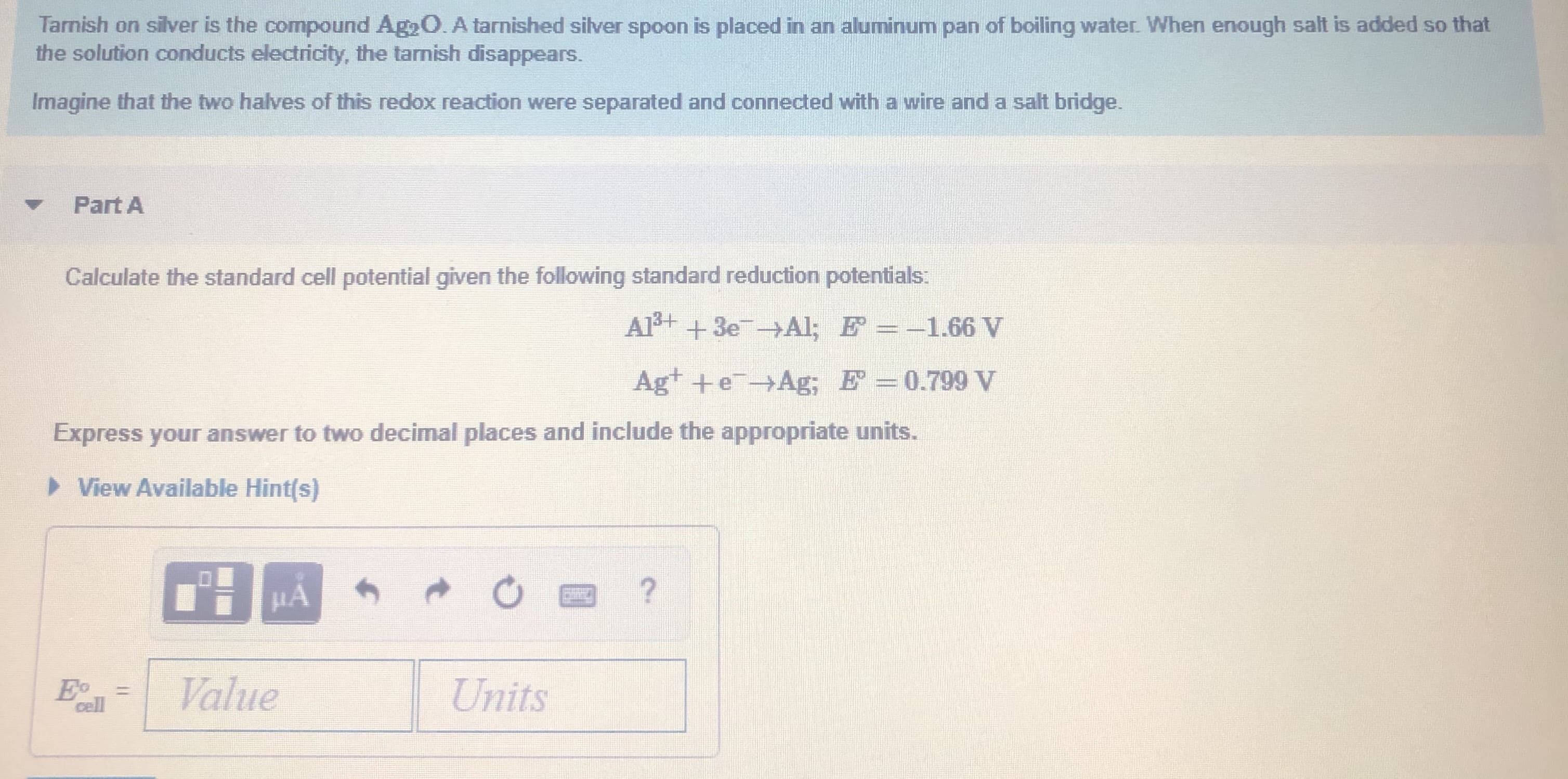
Transcribed Image Text:Tarnish on silver is the compound Ag2O. A tarnished silver spoon is placed in an aluminum pan of boiling water. When enough salt is added so that
the solution conducts electricity, the tarnish disappears.
Imagine that the two halves of this redox reaction were separated and connected with a wire and a salt bridge.
Part A
Calculate the standard cell potential given the following standard reduction potentials:
Al+ +3e Al; E =-1.66 V
Agt +eAg; E =0.799 V
Express your answer to two decimal places and include the appropriate units.
> View Available Hint(s)
Eo
cell
Value
Units
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Recommended textbooks for you

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning