Chapter5: Gases
Section: Chapter Questions
Problem 143CWP: A certain flexible weather balloon contains helium gas at a volume of 855 L. Initially, the balloon...
Related questions
Question
Help me to answer this:
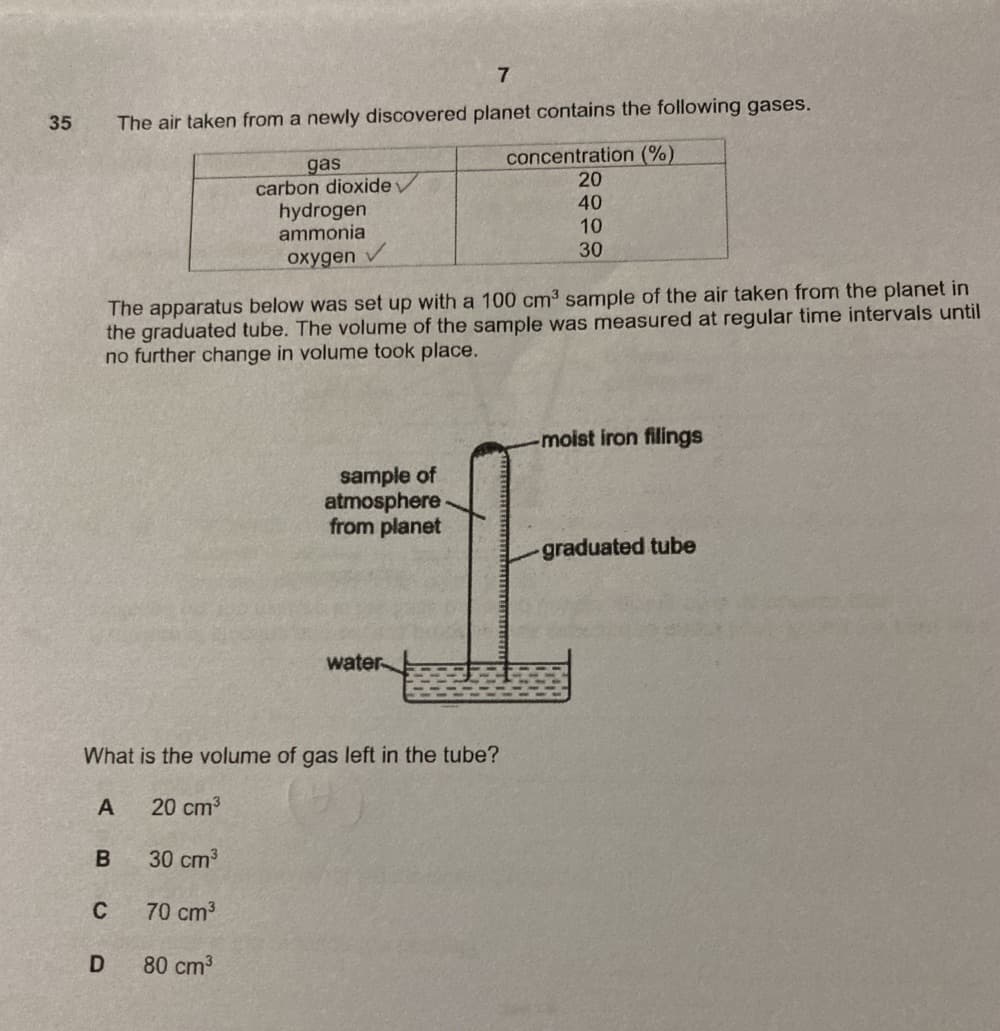
Transcribed Image Text:7
35
The air taken from a newly discovered planet contains the following gases.
concentration (%)
20
gas
carbon dioxide
hydrogen
ammonia
40
10
30
охудen
The apparatus below was set up with a 100 cm3 sample of the air taken from the planet in
the graduated tube. The volume of the sample was measured at regular time intervals until
no further change in volume took place.
-moist iron filings
sample of
atmosphere
from planet
graduated tube
water
What is the volume of gas left in the tube?
A
20 cm3
B
30 cm3
70 cm3
80 cm3
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 2 images

Recommended textbooks for you


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,