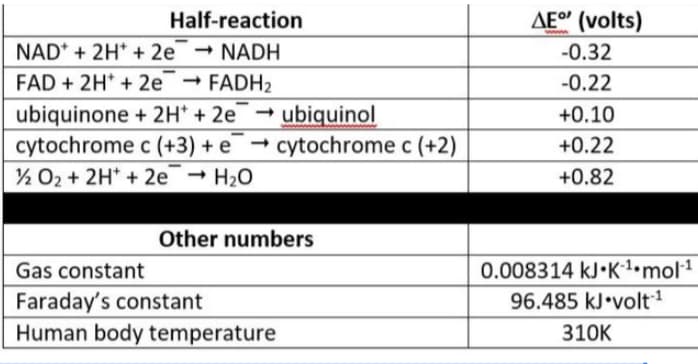
Using the data in this table, what is the AG° (in KJ/mol) for the reduction of FAD by water
Q: Match the following methods of Analysis Fractional analysis, methylation, and periodate oxidation A.…
A: Methods of analysis are different techniques used to analyse a compound for determination of its…
Q: Pyruvate can be processed under anaerobic conditions to ethanol (in yeast) or to lactate (in…
A: Introduction: Pyruvate is the product of glycolysis which is an important junction point in…
Q: Describe surroundings at home which reminds you about biochemistry and relate the situation to…
A: Food is the source of macronutrients and micronutrients required for the body. During the process of…
Q: What are the structural features of 7N7H (Viperin-like enzyme)? a) Must show 3D structure
A: An enzyme is a biological catalyst. it is usually protein. 'Viperin' is a member of the radical…
Q: composition of inactive spliceosome?
A: The five splicing snRNPs and other proteins involved in splicing assemble on a pre-mRNA, forms the…
Q: The following enzymes are involved in carbohydrate digestion in the microvilli, except?
A: Carbohydrate digestion starts with the mouth itself. The salivary glands present in the mouth…
Q: What is the role of decarboxylation in fatty acid synthesis? Describe another process discussed in…
A: Fatty acids are the body's fat-building blocks. Acetyl-CoA is used to make fatty acids. Fatty acid…
Q: If you set up an in vitro translation reaction containing poly(ACGU), as template, which of the…
A: A three letter codon is a sequence of DNA or RNA that corresponds to a specific amino acid.
Q: Why does the adenosine derivative cordycepin inhibit RNA synthesis and how does this support the…
A: The process of transcription involves the synthesis of RNA by DNA dependent RNA Polymerase.
Q: Complete the table below by supplying AT LEAST ONE example of a monomer with its polymer for each…
A: Lipids are hydrophobic or hydrophilic bio macromolecules which can be of several types like fatty…
Q: What term is typically used to describe gene systems that respond to the supply of a required…
A: These terms are generally used in operon models. An operon is a unit of bacterial gene expression…
Q: Explain the meaning of metabolic interconversion
A: Metabolism is the body's cells is used for changing the food into energy. The human bodies need this…
Q: Briefly describe "Lipids" and give examples
A: Lipids biological macromolecules non-polar molecules that is consist of hydrocarbons. It is soluble…
Q: Explain in detail the role of biotechnology in production of baker’s yeast.
A: For millennia, humans have used the metabolic activity of yeasts in baking and brewing. The creation…
Q: Which of the following statements is TRUE regarding the ABO blood system? People who have the…
A: There are 4 main blood groups A, B, AB and O and each of these groups can be either RhD positive or…
Q: Create a diagram showing the biosynthesis of prostaglandin and leukotrienes in the body.
A: Prostaglandins : These are lipid compounds called as eicosanoids, found in tissues in humans and…
Q: Adeno-associated viral vectors integrate into the genome of non-dividing or slowly dividing cells.…
A: Adenoviruses are non-enveloped viruses with a double-stranded DNA genome and an icosahedral…
Q: Roam around your kitchen and collect some items that you can categorize as sources of each…
A: Carbohydrates are one of the important nutrient. Its major functions as a energy source, and also…
Q: Transport of lipids in blood involve lipoproteins which includes the LDL and HDL commonly referred…
A: Lipoproteins carry fats in the bloodstream. Lipoproteins are made up of an inner core of hydrophobic…
Q: Name of complex Electron donor No. of H+ ions pumped (NADH/FADH2) 1. 5. 7. 2. 6. 8. 3. 9. 4. 10.
A: The electron transport chain occurs in the mitochondria. There are four electron transport chain…
Q: RNA Pol III transcribes the following class of RNAS TRNAS rRNAs small nuclear RNAS All of the above
A: RNA pol III :- eukaryotic RNA polymerase, help in eukaryotic transcription.
Q: Assume you were given a mixture consisting of one molecule each of all possible sequences of a…
A: Proteins are long chains of single amino acids. The human body uses 20 different amino acids in…
Q: Which of the following statements are TRUE? Multiple answers:Multiple answers are accepted for…
A: The TCA cycle or tricarboxylic acid cycle is the second stage of cellular respiration. This is a…
Q: 19. For the helix in double-stranded B-form DNA, the majority of the stability of the DNA can be…
A: Hi! Thank you for the question, as per the honor code, we are allowed to answer the…
Q: TRNA and 5S RNA genes both have the following internal control region. BoxA BoxB BoxC
A: An internal control region is determines as a sequence that is made up of DNA and is located only…
Q: According to the Arrenhius theory, an acid is: a. a substance that forms hydroxide ions b. a…
A: Arrhenius theory gives us the concept of acid and base. This theory is given based on the…
Q: The structure below is a он NH но HO "он cerebroside monoglycosyl ceramide glycosphingolipid all are…
A: Lipids are a macro biomolecules made of fatty acid monomers, naturally occurring organic compounds…
Q: What is the difference between gain of function and loss of function mutations?
A: Mutations is a change in a DNA sequence that can be caused due to DNA replication error made during…
Q: Acetyl CoA + 2H* + 2e = pyruvate + COASH Ubiquinone + 2H* + 2e = Ubiquinol E* = -0.48 V E" = +0.04 V…
A: If the reaction has a positive value of standard cell potential/standard reduction potential or a…
Q: What is the process in which antibodies attach to antigens, causing the formation of masses of…
A: Because the Y-shaped antibody arms randomly attach to many surfaces of non-self red blood cells,…
Q: The pyruvate S lactate reaction in animals is reversible, but the pyruvate S ethanol fermentation in…
A: Pyruvate to lactate reaction takes place in our muscle or other microorganisms while fermentation…
Q: Name and discuss the non-covalent interactions that maintain protein structure.
A: Several noncovalent interactions stabilize the three dimensional structure of a protein and hence…
Q: 1. How much faster is a reaction with the faster enzyme than without a catalyst? * A. Approximately…
A: “Since you have asked multiple question, we will solve the first question for you. If youwant any…
Q: How does compromised pyruvate kinase activity lead to anemia?
A: Pyruvate kinase is a catalytic enzyme that catalyzes the final step of glycolysis, which is crucial…
Q: Match the following descriptions with the correct lipid-based compounds:…
A: Introduction: Lipids are a heterogeneous group of biomolecules that include fats, oils, waxes, and…
Q: D-galactose from B-D-glucose can be differentiated using which method of analysis?
A: Glucose and galactose are carbohydrates. Galactose and glucose are examples of monosaccharides.…
Q: What compounds remain in the ethanol solution after the DNA is removed bt spooling onto a glass rod?
A: Introduction: The extraction of DNA is the first step for subsequent molecular or forensic…
Q: draw the full equation for this triacylglycerol undergoing saponification, using KOH.
A: In the process of saponification, triglycerides are reacted with sodium or potassium hydroxide to…
Q: Germline editing affects only somatic cells. True False
A: In addition to connective tissue, blood, bones, and internal organs, somatic cells also make up the…
Q: NADH produced in glycolysis is transported to the mitochondria where the electron is transferred to…
A: Glycolysis is also known as the Embden Meyerhof pathway and it is highly conserved from humans…
Q: Yeast fermentation is of a value in making baked products due to the release of: Select one: O a.…
A: Fermentation occurs in the yeast in anaerobic conditions. Alcoholic fermentation occurs in the…
Q: Lipids are water-soluble substances that tend to form surface monolayers or micelles.
A: Lipids are a class of molecules which have very poor water solubility, by definition. So, the…
Q: 3. Which of the following is not a part of relative substrate specificity? a) Group specificity b)…
A: Enzymes are proteins that assist the bodies speed up chemical processes. Enzymes act on substrate…
Q: running the reaction at 83 °C cooling the reaction to 11 °C changing the pH to 5.4 Increase reaction…
A: The rate of an enzyme catalyzed reaction depends on various factors like temperature and pH of the…
Q: How would you develop an assay for soybean trypsin inhibitor and use it to test products??
A: Trypsin inhibitor: Also known as serine protease inhibitor i.e serpins inhibitors control the…
Q: The residual end of a pre-mRNA transcript is digested by the following enzyme
A: Transcription is the process of formation of pre-mRNA (precursor mRNA) (heterogenous mRNA) from the…
Q: What is the purpose of acrylamide and bis-acrylamide? in SDS-Page Gel
A: Sodium dodecyl sulphate-Polyacrylamide gel electrophoresis (SDS-PAGE) is a common analytical…
Q: Lec 3 Protein Digestion Make a flow chart of: (Indicate the organs, enzymes and how it functions)…
A: In process of digestion, complex molecules are converted to simple molecules with the…
Q: Genetically modified cotton plants produce the toxin encoded from the gene originated from the…
A: Any organism whose genetic material has been altered using genetic engineering techniques is…
Q: The DNA and associated proteins of a eukaryotic chromosome are called Chromatin Chromatosome…
A: Eukaryotic chromosomes are made up of DNA that is tightly coiled around histone protein clusters.…
Using the data in this table, what is the AG° (in KJ/mol) for the reduction of FAD by water?
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

- The Effect of lodoacetic Acid on the Glyceraldehyde-3-P Dehydrogenase Reaction (Integrates with Chapters 4 and 14.) How might iodoacetic acid affect the glyceraldehydes-3-phosphate dehydrogenase reaction in glycolysis? Justify your answer.Energetics of the Hexokinase Reaction The standard-state free energy change. Gfor the hexokinase reaction, is — 1 6.7 kJ/mol. Use the values in Table I to calculate the value of Gfor this reaction in the erythrocyte at 37°C.Complete oxidation of a 16-carbon fatty acid can yield 129 molecules of ATP Study Figure 19.2 and determine how many ATP molecules would be generated if a 16-carbon fatly acid were metabolized solely by the TCA cycle, in the form of S acetyl-CoA molecules.
- The Relative Efficiency of ATP Synthesis in Noncyclic versus Cyclic Photophosphorylation If noncyclic photosynthetic electron transport leads to the translocation of 7 H+/2e- and cyclic photosynthetic electron transport leads to the translocation of 2 H+/e-, what is the relative photosynthetic efficiency of ATP synthesis (expressed as the number of photons absorbed per ATP synthesized) for noncyclic versus cyclic photophosphorylation? (Assume that the CF1CF0-ATP synthase yields 3 ATP/14 H+.)Calculate the biochemical standard cell potential for the oxidation of NADH by molecular oxygen O2 + 2NADH + 2 H+ → 2H2O + 2NAD+a) Calculate the enzyme and specific activity of a reaction with 3 μM Hsp90 using the following information: The rate is measured in a spectrophotometer as 0.028 OD units/min in a 1 ml reaction volume. The absorbance was detected at 340nm and the extinction coefficient for NADH at this wavelength is 6200 L M-1 min-1 and the molecular mass of Hsp90 is 82.7 kDa. The rate of NADH utilisation is equivalent to the rate of ATP utilised by Hsp90. Show all your calculations and the units for your answers. b) Calculate the turnover number for the reaction described in (a) above
- The standard reduction potential for ubiquione (A or coenzyme Q) is .045 V, and the standard reduciton potential (E) for FAD is -0.219 V. Using these values, show that the oxidation for FADH2 by ubiquinone theoretically liberates enough energy to drive the synthesis of ATP. Faraday constant =96.48KJ/Vol delta G' standard for ATP Synthesis is +30.5 KJ/mol R=8.314 J/mol K=1.987 cal/mol KCalculate the ΔG for Malate dehydrogenase reaction of CAC if the concentration of oxaloacetate is 1x 10-8 M, malate is 0.2 mM, NAD+ is 10 mM and NADH is 0.1 mM in rat liver mitochondria. ΔGo’ for this reaction is 30 kJ/mole. Please write the units of the final answer and the formula you are using to solve. R = 0.008314kJ/K*mol, T = 298KComplete the balanced equation for the overall reaction. Select answer choice in between brackets. Sucrose + [2 Pi, 4Pi]+[4 ADP, 2 ADP, 4 ATP, 2ATP]+[2 NAD+, 4 NAD+, 6 NAD+]+[H2O, 5 H2O, 3 H2O] --> [2 cirate, 2 oxaloacetate, 2 pyruvate, 2 acetyl-coA]+[4 ADP, 2 ADP, 4 ATP, 2ATP] + [2 NAD+, 4 NAD+, 6 NAD+] + [2H+, 8H+, 6 H+, 4 H+, 10 H+] Does the commercial process require aerated culture medium—that is, is this a fermentation or an aerobic process? A. a fermentation process, because A. niger cells must use O2O2 to continuously regenerate NAD+ B. an aerobic process, because A. niger cells must use O2O2 to continuously regenerate NAD+ C. a fermentation process, because A. niger cells cannot use O2O2 to continuously regenerate NAD+ D. an aerobic process, because A. niger cells cannot use O2O2 to continuously regenerate NAD+
- One process catalyzed by NADHNADH dehydrogenase is NADH+H^++ubiquinone ↽−−⇀ NAD+ubiquinolNADH+H^++ubiquinone ↽−−⇀ NAD^++ubiquinol The standard reduction potentials for the half‑reactions are given in the table. Oxidant Reductant ?′0 ubiquinone+2H++2e−ubiquinone+2H++2e^− ubiquinolubiquinol 0.045 NAD^++H^++2e−NAD^++H^++2e^− NADHNADH –0.32 Calculate Δ?′0 for the reaction as shown. Δ?′0=____(V) Calculate Δ?′0 . Δ?′0=____(kJ/mol)Complete the balanced equation for the overall reaction by selecting an answer choice in the brackets. Sucrose + [2 Pi, 4Pi]+[4 ADP, 2 ADP, 4 ATP, 2ATP]+[2 NAD+, 4 NAD+, 6 NAD+]+[H2O, 5 H2O, 3 H2O] --> [2 cirate, 2 oxaloacetate, 2 pyruvate, 2 acetyl-coA]+[4 ADP, 2 ADP, 4 ATP, 2ATP] + [2 NAD+, 4 NAD+, 6 NAD+] + [2H+, 8H+, 6 H+, 4 H+, 10 H+] Does the commercial process require aerated culture medium—that is, is this a fermentation or an aerobic process? A. a fermentation process, because A. niger cells must use O2O2 to continuously regenerate NAD+ B. an aerobic process, because A. niger cells must use O2O2 to continuously regenerate NAD+ C. a fermentation process, because A. niger cells cannot use O2O2 to continuously regenerate NAD+ D. an aerobic process, because A. niger cells cannot use O2O2 to continuously regenerate NAD+Most biochemists agree that the most accurate number of H+ needed to generate one ASP is blank. Therefore one NADH generated about blank ATP molecules?

