The boiling point composition diagram for a binary mixture of benzene and isopropanol is shown below: 82.0 80.0 78.0 76.0 74.0 72.0 70.0 0.0 0.20 0.40 0.60 0.80 1.00 Xisopropanol (a) What is the boiling point of pure isopropanol? (b) What is the boiling point of the solution when Xjsopropanol = 0.90? (c) What is the composition of the vapor immediately above the solution in part b? Boiling Point (°C)
The boiling point composition diagram for a binary mixture of benzene and isopropanol is shown below: 82.0 80.0 78.0 76.0 74.0 72.0 70.0 0.0 0.20 0.40 0.60 0.80 1.00 Xisopropanol (a) What is the boiling point of pure isopropanol? (b) What is the boiling point of the solution when Xjsopropanol = 0.90? (c) What is the composition of the vapor immediately above the solution in part b? Boiling Point (°C)
Chemistry: An Atoms First Approach
2nd Edition
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Steven S. Zumdahl, Susan A. Zumdahl
Chapter10: Properties Of Solutions
Section: Chapter Questions
Problem 7RQ
Related questions
Question
can you help with sub questions A, B and C
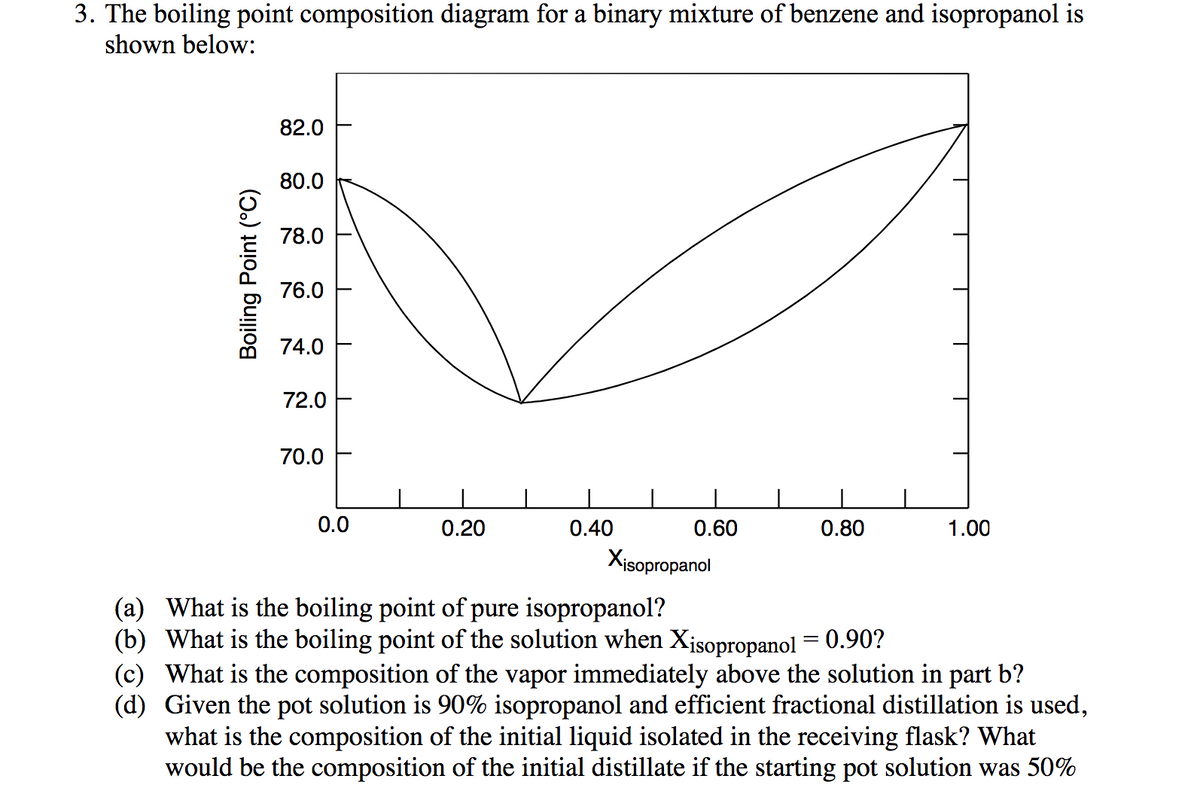
Transcribed Image Text:3. The boiling point composition diagram for a binary mixture of benzene and isopropanol is
shown below:
82.0
80.0
78.0
76.0
74.0
72.0
70.0
0.0
0.20
0.40
0.60
0.80
1.00
Xisopropanol
(a) What is the boiling point of pure isopropanol?
(b) What is the boiling point of the solution when Xisopropanol = 0.90?
(c) What is the composition of the vapor immediately above the solution in part b?
(d) Given the pot solution is 90% isopropanol and efficient fractional distillation is used,
what is the composition of the initial liquid isolated in the receiving flask? What
would be the composition of the initial distillate if the starting pot solution was 50%
Boiling Point (°C)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Recommended textbooks for you

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:
9781305446021
Author:
Lampman
Publisher:
CENGAGE LEARNING - CONSIGNMENT

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning