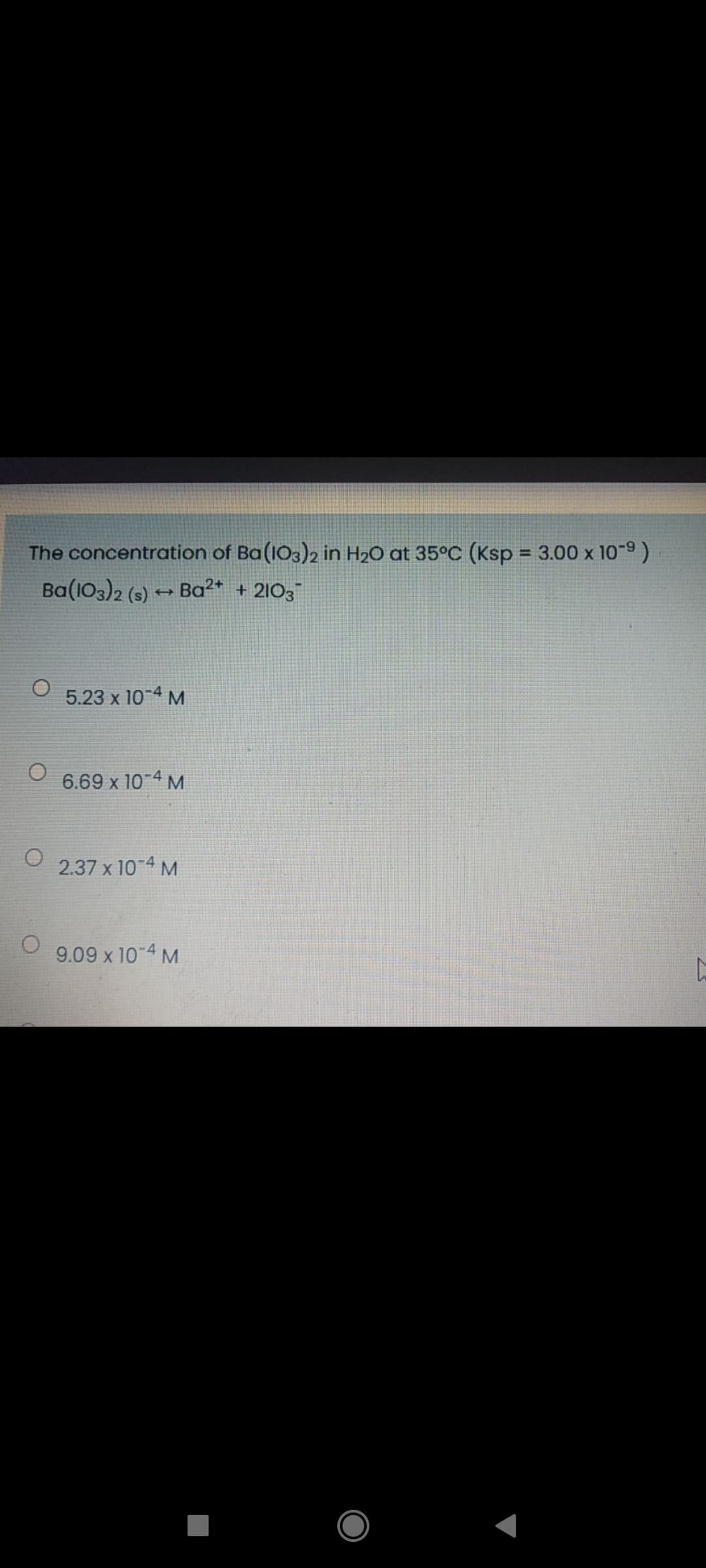
The concentration of Ba(103)2 in H2O at 35°C (Ksp = 3.00 x 10- %3D Ba(103)2 (s) - Ba²* + 2103 5.23 x 10-4 M 6.69 x 10-4 M 2.37 x 10 4 M 9.09 x 10-4 M
Q: 1. What's the slope of the calibration curve? 3.95 x 10-5 4.17x10-5 1 20484 25257 2. What's the…
A: The linear regression equation of the calibration curve is: Y = 25257X - 114.32
Q: Pre-lab question #6-1: Calculate the percent (%) C2042- in H2C204. 53.0 57.1 65.7 97.8
A: Mass percent: Mass percent of ion = atomic mass of the ionMolar mass of the compound×100
Q: Separation of individual components from a multi-component mixture can best be carried by Solvent…
A:
Q: (2) j lubäi On the gravimetric determination of CI, if the weight of AgCl ppt. was 1.433 g.; the…
A: This method is used for the determination of the analyte based on its mass. The principle behind the…
Q: What is the maximum metallic concentration in 100.0 liters of water from a metallic hydroxide with a…
A:
Q: You obtained the following raw data when setting up a Bradford standard curve: BSA (mg/ml)…
A: First, we have to find the corrected absorbance values by subtracting the absorbance of each value…
Q: As the temperature of a gas chromatography column increases, the the retention time of the analyte…
A: Gas chromatography is a technique used for the separation of compounds and analyzing the compound…
Q: 10. Given: PtCl4- (aq) + 2e · Pt(s) + 4 CI (aq) eº = U.755 V Pt2*(aq) + 2e¯ -> Pts) e° = 1.188 V…
A: The question is based on the concept of electrochemistry. we have to calculate formation constant…
Q: You obtained the following raw data when setting up a Biuret standard curve: Absorbancy 540nm BSA…
A: A question based on tools in analytical chemistry that is to be accomplished.
Q: 5. Using the extinction coefficients below, calculate the percent yield using the Amax- Wavelength…
A:
Q: I'm not sure how to solve for Molarity of NaOH and average molarity of NaOH
A: Molarity = no of moles per unit volume Given Volume.of acid = 25 mL Molarity of acid = 0.0762 M…
Q: A student wanted to determine the level of lead in amoxicillin powder, so he transferred 148 mg of…
A: Given the absorbance of the diluted solution, Y = 0.48 Calibration equation is: Y = 12.25X - 0.05…
Q: What is the Ksp of Zn3(PO4)2 (MW = 320.8 g/mol) if its solubility in water at 25 °C is 4.9 x 10-5…
A:
Q: Barium sulfate from a 1.300-g sample was contaminated with 9.4 mg of Fe2(SO4)3 and weighed a total…
A: Solution - According to the question - Given - Molar weight of BaSO4 = 233.38 g/mol Mass of sample =…
Q: A student wanted to determine the level of lead in amoxicillin powder, so he transferred 148 mg of…
A: Given: The absorbance of the diluted solution, y = 0.48 Calibration equation is given by y = 12.25…
Q: The pNa of a mixture prepared by mixing 50 ml of 0.00063 M of NaCl and 300 ml of 0.00040 M in Na3PO4…
A: We know, pNa = -log([Na+]) ---> (1) Molarity = moles/Volume --> (2) Millimoles =…
Q: The pNO; in a solution that is 567 ppm in Zn(NO;)2 and 3.0 x 10-3 M Cd(NO3)2 are ....... A.W Zn=65,…
A:
Q: Question attached
A:
Q: 3H, t SH, multiple 2H, 4 10 6. 80 5. 4 3 2 HSP-06-296 ppm రబో A E
A:
Q: A sample of anhydrous NaHCO3 (FM = 84.007) is suspected to be contaminated with either NaOH (FM =…
A: NaHCO3 is present as the Impurity. Now, mass of Na2CO3 Present = 27.2×10-3×0.0125×4×5×105.989 =…
Q: In which of the following mixture(s) would CaF2(s) (Ksp = 4.0 x 10-11) form? 30 mL of 0.00020…
A:
Q: How many moles of calcium fluoride (78.07 g/mol) will dissolve in 10.0 L of water at 25°C? Ksp of…
A:
Q: & protein in a solution measured in a 96 well plate You have the following information: volume in…
A:
Q: Find the ksp of PbCl2 5.0 g od pbcl2 100ml of h2o
A: GIVEN DATA: given mass of PbCl2: 5gm Molar mass of PbCl2 is 278.1gmol-1 water = 100ml
Q: a.) 1.00g KCI in 75.0mL of soutin = b.) 1.00g NazcrOy in 75.0mL of Selutiin= C.) 20.0g MaBrz in…
A: Molar Concentration of the following solutions can be calculated as -
Q: 7. A sample of anhydrous NaHCO; (FM = 84.007) is suspected to be contaminated with either NaOH (FM =…
A:
Q: Flask 1 2 3 Flask 1 2 3 initial burette reading 50 mL. 38 mL 28 mL volume EDTA required to chelate…
A: The explanation is given below-
Q: A titrimetric method for the determination of calcium in limestone was tested by analysis of an NIST…
A: The confidence interval can be calculated using the following formula:
Q: emistry R122 dy Assignment 3 Solubility Equilibria How many grams of Ca3(PO4)2 will dissolve in a)…
A: The solubility product of calcium phosphate is equal to 2×10-29. Let the number of moles of calcium…
Q: I am confused on how to do one and two? Help please.
A: We are authorized to answer one question at a time. Please repost your remaining questions…
Q: 26. In a certain paper chromatography experiment, the Ri values of components X, Y, and Z are 0.25,…
A: Mixture is a type of matter which is composed of two or more substances. These are called as…
Q: What is the percent error of the Ksp of Ca(OH)2 if the experimental value is 9.1 x 10^-5 and the…
A: Given Experimental value = 9.1 * 10-5 Accepted value = 5.5 * 10-6
Q: A student determines that the value of Ka for H2SO3 = 2.3×10-2 . What is the value of pKa?
A: pKa is the measure of determining the strength of the acid. Lower the value of pKa, stronger will be…
Q: Prepare 2ppm, 4 ppm, 6ppm, 8ppm, 10 ppm of Cd(NO3). 4H2O in a 500ml
A:
Q: Calculate the water solubility of H2CO3for water in contact with 1000 ppm of CO2(1000 x 106atm;…
A: Henry's law describes that the amount of dissolved gas in a solution is directly proportional to the…
Q: In the determination of Ca2+ in milk powder, 12.1 mL 0.01 M EDTA is used for 1.50 grams of sample.…
A:
Q: 6. In a flame spectrometric determination of sodium, lithium is used as an internal standard. Assume…
A: The concentration and intensity are directly related to each other. According to Beer's-Lambert…
Q: QUESTION. a.) What is the concentration of the HClO₄ solution? b.) What is the concentration of the…
A: Given : Mass of Na2CO3 sample = 0.2119 gm Volume of HClO4 = 50.0 ml Volume of NaOH used = 10.28…
Q: Calculate the solubility of ZnC204 in a solution held at pH 3.00. Ksp (ZnC204) = 7.5´ 10-9 1.8 10-10…
A:
Q: Calculate initial concentrations added to the test tube. (Fe+3 and SCN-1) 5 mL of 0.002 M Fe(NO3)3,…
A:
Q: The concentration of Ba(103)2 in H2O at 30°C (Ksp = 1.25 x 10-) Ba(10s)2 (s) Ba" + 2103 7.32 x 10-8…
A: Given, Ksp of Ba(IO3)2= 1.25×10-9
Q: 4- A sample containing H2C204 had a purity equal to 90.50% (w/w). An unknown mass of this sample was…
A:
Q: The protein concentration of a protein isolate was determined using the Bradford assay. Five…
A: A question based on tools in analytical chemistry that is to be accomplished.
Q: 3.) The following Beer's Law calibration plot was collected at 391.9 nm using the five mixtures…
A: We can use the given trend line equation for Absorbance versus chromate ion concentration graph and…
Q: Prepare 2ppm,4ppm, 6ppm, 8ppm and 10ppm of Cd (NO3).4 H2O in a 100ml
A: Given that - Volume of Solution of cadmium nitrate tetrahydrate , Cd(NO3)2 .4H2O = 100 mL And,…
Q: 1) a)What is affinity chromatography and what is it used for? b) A drug called Bamlanivimab is…
A:
Q: In a Bradford assay, 11 µL of a protein isolate sample was diluted by adding 21 µl of water prior to…
A: Solution- Data given- 11 µL of protein + 21 µL water , BSA protein conc. = 200 µg/ml To find-…
Q: The Ksp of Mg(OH)2 is 5.61 x 10-12, If you tried to dissolve 24.0 mg of Mg(OH)2 in 250 mL of wąter…
A: I think your question is incorrect. It should be ask the amount of dissolved solid according to…
Q: Determination of Ksp of CaCO3 based on the measured pH and the following equations: CaCO3 (s) Ca 2+…
A: Here in this question some informations are given (Concentration value of Ca2+and PH value of the…
Q: Which of the following methods is mod suitable for the purification of new solid organic 37 compound…
A: Given Technique A. Evaporation B. fractional Distillation C. Steam Distillation D. Recrystallization…
Step by step
Solved in 2 steps with 1 images

- The Ksp of Ca(OH)2 is 1.5*10^-10. What is the concentration of OH?The Ksp for BaF2 is 1.6x10^-6.What is tge concentration of Ba+2 ions in this solution?17. The Ksp for Zn3(AsO4)2 is 1.1 ×10−27. When 100 mL of 5.5 ×10−4 M Zn2+ is mixed with 50 mL of 1.2 × 10−4M AsO43−, which of the following statements is true? What is the new concentration of Zn2+? a 2.53 x 10-5M b 4.91 x 10-5M c 5.32 x 10-4M d 3.67 x 10-4M
- The Ksp of Fe(OH)3(s) is 3 ×10-39. What concentration of Fe3+ can exist in pure water? A. 3 ×10-4 M B. 1 ×10-10 M C. 3 ×10-21 M D. 3 × 10-24 M E. 3 × 10-30 Mavg concentration: 8.937x10^-7The solubility of the related salt hydroxyapatite, Ca5(PO4)3OH (Ksp = 1 x 10-29), is affected by the pH of the water to which it is added. a) Calculate the number of grams of hydroxyapatite that will dissolve in 0.250L of neutral water. b) Calculate the number of grams of hydroxyapatite that will dissolve in 0.250L of water having a pH = 5.5
- The Ksp value for magnesium arsenate [Mg3(AsO4)2] is 2.00 X 10-20. Suppose a chemist mixed 33.23 mg of Mg2+(aq) with 44.56 mg of AsO43-(aq) in 21.47 Liter of water. Which way will the equilibrium shift? Question 10 options: right left then right left at equilibrium then right at equilibrium then left not enough information at equilibrium right then left can't be determinedConsider the titration illustrated in Figure 8.] Anhydrous sodium carbonate (Na₂CO₃) is a primary standard. When 0.364 grams of the substance is placed in a conical flask, then 20.00 cm³ of sulphuric acid (H₂SO₄) solution is required to reach the end point of the titration. What is the concentration in mol·dm⁻³ of the sulphuric acid solution? [Give the answer to 3 decimal places. Do not type in the unit. Use a decimal point.] *Yttrium (III) carbonate (MM = 357.84 g/mol) has a Ksp of 1.0 x10-31. If 15.5 g Y2(CO3)3 is stirred into 2.16 L H2O, how many micrograms of yttrium (III) carbonate will dissolve?Report your answer to the nearest whole number.
- Ksp for BaCrO4 is 1.17x10^-103 The atmosphere in a workplace contains 300 ppm of acetone (TLV 1000 ppm),125 ppm of ethyl acetate (TLV 400 ppm) and 65 ppm of methyl ethyl ketone (TLV 200 ppm). What is the combined effect of this hazardous substances and is it acceptable and explain your answer.The solubility of Mg(OH)2 in water is 1.4 x 10^-4 at 25C. What is the Ksp?

