The decalinols A and B can be equilibrated using aluminum isopropoxide in 2-propanol (isopropyl alcohol) containing a small amount of acetone. Al[OCH(CH,),1, НО acetone ОН A B Assuming a value of AG° (equatorial to axial) for cyclohexanol is 4.0 kJ (0.95 kcal)/mol, calculate the percent of each decalinol in the equilibrium mixture at 25°C.
The decalinols A and B can be equilibrated using aluminum isopropoxide in 2-propanol (isopropyl alcohol) containing a small amount of acetone. Al[OCH(CH,),1, НО acetone ОН A B Assuming a value of AG° (equatorial to axial) for cyclohexanol is 4.0 kJ (0.95 kcal)/mol, calculate the percent of each decalinol in the equilibrium mixture at 25°C.
Organic Chemistry
8th Edition
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Chapter5: Alkenes: Bonding, Nomenclature, And Properties
Section: Chapter Questions
Problem 5.31P
Related questions
Question
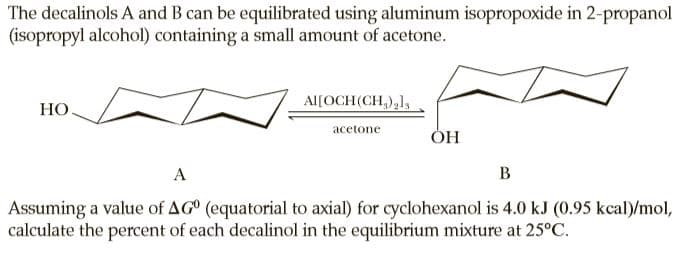
Transcribed Image Text:The decalinols A and B can be equilibrated using aluminum isopropoxide in 2-propanol
(isopropyl alcohol) containing a small amount of acetone.
Al[OCH(CH,),1,
НО
acetone
ОН
A
B
Assuming a value of AG° (equatorial to axial) for cyclohexanol is 4.0 kJ (0.95 kcal)/mol,
calculate the percent of each decalinol in the equilibrium mixture at 25°C.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Recommended textbooks for you

Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning

Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning