The enzyme cytidine deaminase catalyzes the conversion of cytidine to uridine. Cytidine deaminase catalyzes the reaction through an addition of water across the cytidine 3,4-bond, forming a tetrahedral intermediate followed by the elimination of NH3 to form the product uridine. This is like the addition-elimination mechanism that we studied for adenosine deaminase. Cytidine deaminase HO. NH₂ OH OH cytosine N I R N + H₂O cytidine ΝΗ uridine The Km value for the substrate cytidine is 2.5 x 10-4 M, and the K₁ for competitive inhibition by the product uridine is 2.5 × 10-³ M. 4 HO. A reduced derivative of the product, 3,4,5,6-tetrahydrouridine was shown to be a fully reversible competitive inhibitor with a Ki of 2.4 x 10-7 M, a value approximately 10,000 times lower than that of the product uridine. NH₂ 3NH R= D-ribose R uracil ring numbering N HOH OH uridine ΝΗ H HH OH H- H N R + NH3 .H 'N' 3,4,5,6-tetra- hydrouridine a.) Draw a structure of the intermediate that we predict to form during the conversion of cytidine to uridine. b.) Use a mechanistic argument to explain the high inhibitor potency of 3,4,5,6- tetrahydrouridine.
The enzyme cytidine deaminase catalyzes the conversion of cytidine to uridine. Cytidine deaminase catalyzes the reaction through an addition of water across the cytidine 3,4-bond, forming a tetrahedral intermediate followed by the elimination of NH3 to form the product uridine. This is like the addition-elimination mechanism that we studied for adenosine deaminase. Cytidine deaminase HO. NH₂ OH OH cytosine N I R N + H₂O cytidine ΝΗ uridine The Km value for the substrate cytidine is 2.5 x 10-4 M, and the K₁ for competitive inhibition by the product uridine is 2.5 × 10-³ M. 4 HO. A reduced derivative of the product, 3,4,5,6-tetrahydrouridine was shown to be a fully reversible competitive inhibitor with a Ki of 2.4 x 10-7 M, a value approximately 10,000 times lower than that of the product uridine. NH₂ 3NH R= D-ribose R uracil ring numbering N HOH OH uridine ΝΗ H HH OH H- H N R + NH3 .H 'N' 3,4,5,6-tetra- hydrouridine a.) Draw a structure of the intermediate that we predict to form during the conversion of cytidine to uridine. b.) Use a mechanistic argument to explain the high inhibitor potency of 3,4,5,6- tetrahydrouridine.
Biochemistry
9th Edition
ISBN:9781319114671
Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Chapter1: Biochemistry: An Evolving Science
Section: Chapter Questions
Problem 1P
Related questions
Question
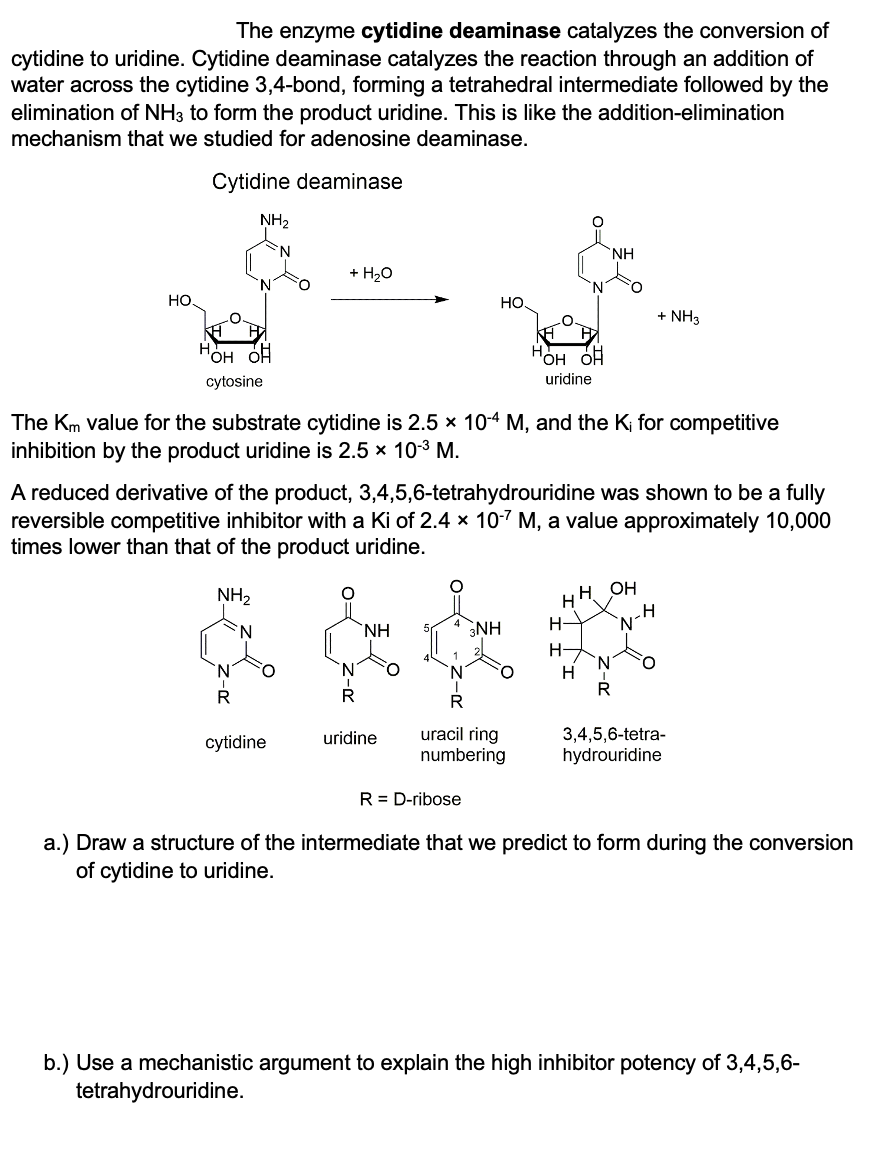
Transcribed Image Text:The enzyme cytidine deaminase catalyzes the conversion of
cytidine to uridine. Cytidine deaminase catalyzes the reaction through an addition of
water across the cytidine 3,4-bond, forming a tetrahedral intermediate followed by the
elimination of NH3 to form the product uridine. This is like the addition-elimination
mechanism that we studied for adenosine deaminase.
Cytidine deaminase
НО.
NH₂
N
пOH OH
cytosine
R
+ H₂O
cytidine
The Km value for the substrate cytidine is 2.5 × 10-4 M, and the K; for competitive
inhibition by the product uridine is 2.5 × 10-³ M.
N
R
A reduced derivative of the product, 3,4,5,6-tetrahydrouridine was shown to be a fully
reversible competitive inhibitor with a Ki of 2.4 x 10-7 M, a value approximately 10,000
times lower than that of the product uridine.
NH₂
NH
uridine
HO.
N
R
R = D-ribose
HOH OH
uridine
3NH
uracil ring
numbering
H
NH
H
H
+ NH3
H OH
H
3,4,5,6-tetra-
hydrouridine
a.) Draw a structure of the intermediate that we predict to form during the conversion
of cytidine to uridine.
b.) Use a mechanistic argument to explain the high inhibitor potency of 3,4,5,6-
tetrahydrouridine.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 4 steps with 2 images

Recommended textbooks for you

Biochemistry
Biochemistry
ISBN:
9781319114671
Author:
Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher:
W. H. Freeman

Lehninger Principles of Biochemistry
Biochemistry
ISBN:
9781464126116
Author:
David L. Nelson, Michael M. Cox
Publisher:
W. H. Freeman

Fundamentals of Biochemistry: Life at the Molecul…
Biochemistry
ISBN:
9781118918401
Author:
Donald Voet, Judith G. Voet, Charlotte W. Pratt
Publisher:
WILEY

Biochemistry
Biochemistry
ISBN:
9781319114671
Author:
Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher:
W. H. Freeman

Lehninger Principles of Biochemistry
Biochemistry
ISBN:
9781464126116
Author:
David L. Nelson, Michael M. Cox
Publisher:
W. H. Freeman

Fundamentals of Biochemistry: Life at the Molecul…
Biochemistry
ISBN:
9781118918401
Author:
Donald Voet, Judith G. Voet, Charlotte W. Pratt
Publisher:
WILEY

Biochemistry
Biochemistry
ISBN:
9781305961135
Author:
Mary K. Campbell, Shawn O. Farrell, Owen M. McDougal
Publisher:
Cengage Learning

Biochemistry
Biochemistry
ISBN:
9781305577206
Author:
Reginald H. Garrett, Charles M. Grisham
Publisher:
Cengage Learning

Fundamentals of General, Organic, and Biological …
Biochemistry
ISBN:
9780134015187
Author:
John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. Peterson
Publisher:
PEARSON