The enzyme Malate Dehydrogenase (MD) catalyzes the conversion of malate to oxaloacetate using icotinamide adenine dinucleotide (NAD) as an enzyme cofactor (or coenzyme). Answer the following questions about this reaction. See pp. 462-467 of your textbook for further information. (a) Briefly explain how we know that this reaction (malate → oxaloacetate) is an oxidation- reduction reaction. (b) Write out the two reductive half reactions and indicate the e°' for each half reaction. Write out the full balanced reaction for the malate to oxaloacetate reaction and indicate the Aeº' for the reaction. (c) What is the free energy change under standard state conditions (AGº') for this reaction? Which direction is spontaneous?
The enzyme Malate Dehydrogenase (MD) catalyzes the conversion of malate to oxaloacetate using icotinamide adenine dinucleotide (NAD) as an enzyme cofactor (or coenzyme). Answer the following questions about this reaction. See pp. 462-467 of your textbook for further information. (a) Briefly explain how we know that this reaction (malate → oxaloacetate) is an oxidation- reduction reaction. (b) Write out the two reductive half reactions and indicate the e°' for each half reaction. Write out the full balanced reaction for the malate to oxaloacetate reaction and indicate the Aeº' for the reaction. (c) What is the free energy change under standard state conditions (AGº') for this reaction? Which direction is spontaneous?
Biochemistry
9th Edition
ISBN:9781319114671
Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Chapter1: Biochemistry: An Evolving Science
Section: Chapter Questions
Problem 1P
Related questions
Question
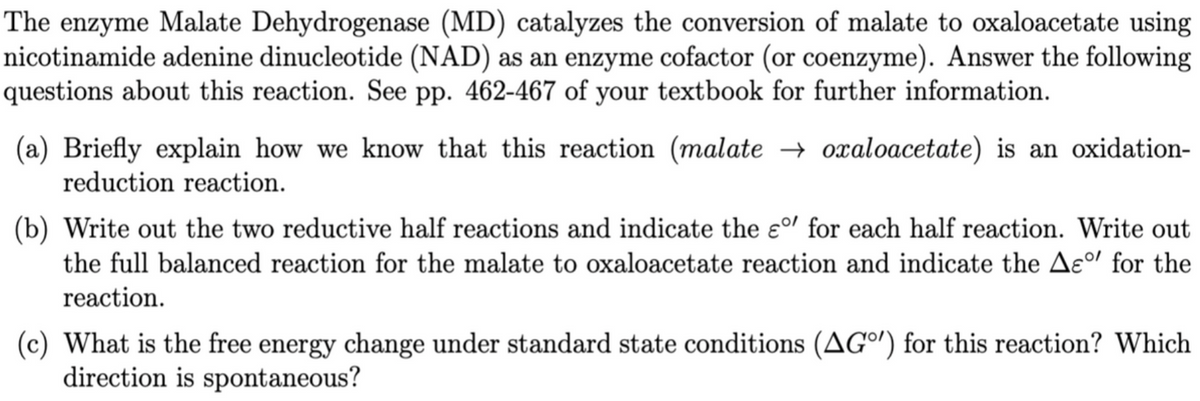
Transcribed Image Text:The enzyme Malate Dehydrogenase (MD) catalyzes the conversion of malate to oxaloacetate using
nicotinamide adenine dinucleotide (NAD) as an enzyme cofactor (or coenzyme). Answer the following
questions about this reaction. See pp. 462-467 of your textbook for further information.
(a) Briefly explain how we know that this reaction (malate → oxaloacetate) is an oxidation-
reduction reaction.
(b) Write out the two reductive half reactions and indicate the ɛº' for each half reaction. Write out
the full balanced reaction for the malate to oxaloacetate reaction and indicate the Ae°l for the
reaction.
(c) What is the free energy change under standard state conditions (AGº') for this reaction? Which
direction is spontaneous?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps

Recommended textbooks for you

Biochemistry
Biochemistry
ISBN:
9781319114671
Author:
Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher:
W. H. Freeman

Lehninger Principles of Biochemistry
Biochemistry
ISBN:
9781464126116
Author:
David L. Nelson, Michael M. Cox
Publisher:
W. H. Freeman

Fundamentals of Biochemistry: Life at the Molecul…
Biochemistry
ISBN:
9781118918401
Author:
Donald Voet, Judith G. Voet, Charlotte W. Pratt
Publisher:
WILEY

Biochemistry
Biochemistry
ISBN:
9781319114671
Author:
Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher:
W. H. Freeman

Lehninger Principles of Biochemistry
Biochemistry
ISBN:
9781464126116
Author:
David L. Nelson, Michael M. Cox
Publisher:
W. H. Freeman

Fundamentals of Biochemistry: Life at the Molecul…
Biochemistry
ISBN:
9781118918401
Author:
Donald Voet, Judith G. Voet, Charlotte W. Pratt
Publisher:
WILEY

Biochemistry
Biochemistry
ISBN:
9781305961135
Author:
Mary K. Campbell, Shawn O. Farrell, Owen M. McDougal
Publisher:
Cengage Learning

Biochemistry
Biochemistry
ISBN:
9781305577206
Author:
Reginald H. Garrett, Charles M. Grisham
Publisher:
Cengage Learning

Fundamentals of General, Organic, and Biological …
Biochemistry
ISBN:
9780134015187
Author:
John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. Peterson
Publisher:
PEARSON