The firstonder is omerization reaetion cy clo propane propere 110 x 10 5.70 x10-4s-l certu adion erergy, Ea for te reaetion? has anate co nslant of at 470°C at 500°C. what is the and 470°C - 743.15K. %3D l.10 x 10-4 g-l Tz = 500°C -773.1 5K In Ki %3D XR=Ea K2- 5.70 (0 45-1 %3D Eq=?
The firstonder is omerization reaetion cy clo propane propere 110 x 10 5.70 x10-4s-l certu adion erergy, Ea for te reaetion? has anate co nslant of at 470°C at 500°C. what is the and 470°C - 743.15K. %3D l.10 x 10-4 g-l Tz = 500°C -773.1 5K In Ki %3D XR=Ea K2- 5.70 (0 45-1 %3D Eq=?
Chapter7: Statistical Data Treatment And Evaluation
Section: Chapter Questions
Problem 7.9QAP
Related questions
Question
I got the same answer but in negative… is it positive or negative and why ? Thank you
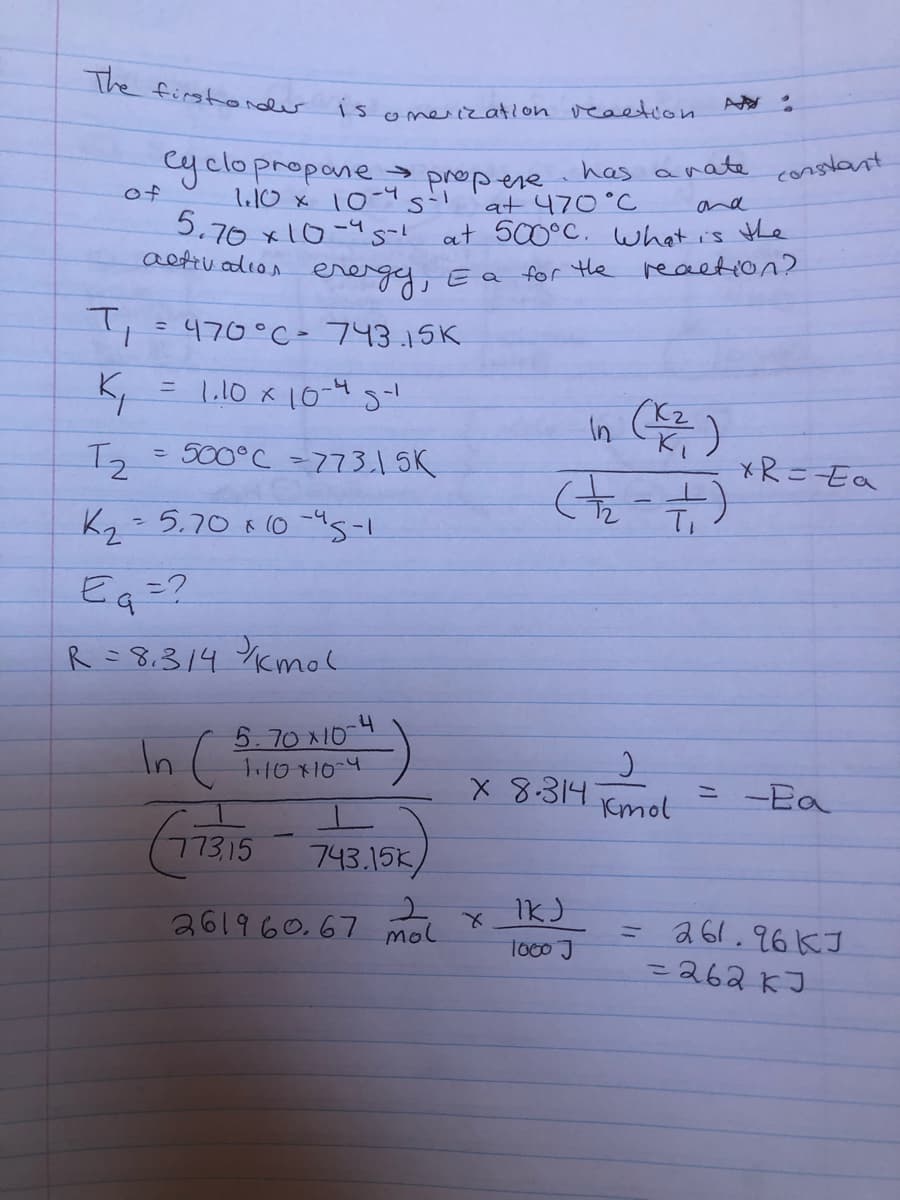
Transcribed Image Text:The firstondr
1somerization veation
cyclopropone
l10 x 10 s
5.70 x10-4s-!
aetiv adion
->
has
nate
constant
propere
at 470°C
at 500°C. what is the
of
and
energy,
E a for the reaction?
470°C - 743.15K.
= L,10 x 10-4 s-!
In )
= 500°C -773.15K
*R=Ea
Kg - 5.70 8 (0 -45-1
Eq=?
R =8.314 Ykmol
5.70x10
In (
X 8.314
Kmol
= -Ea
CTRIS
17315
743.15K
261960.67 mol
= a61.96KJ
=262kJ
To00J
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you



Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning



Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co