-] In the case of polyacene molecules (i.e., C4n+2H2n+4), Tt electrons of the molecules can be treated as being delocalized along their circular paths. For the first few polyacene molecules, absorption regions are as follows: 7. Molecule Absorption region Benzene 255 nm Naphthalene 315 nm Anthracene 380 nm Naphthacene 480 nm 580 nm Pentacene Briefly explai the effects of a red shift (of absorption region) on chemical stability.
-] In the case of polyacene molecules (i.e., C4n+2H2n+4), Tt electrons of the molecules can be treated as being delocalized along their circular paths. For the first few polyacene molecules, absorption regions are as follows: 7. Molecule Absorption region Benzene 255 nm Naphthalene 315 nm Anthracene 380 nm Naphthacene 480 nm 580 nm Pentacene Briefly explai the effects of a red shift (of absorption region) on chemical stability.
Organic Chemistry: A Guided Inquiry
2nd Edition
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Andrei Straumanis
ChapterL3: Carbon (13c) Nmr Spectroscopy
Section: Chapter Questions
Problem 21CTQ
Related questions
Question
5
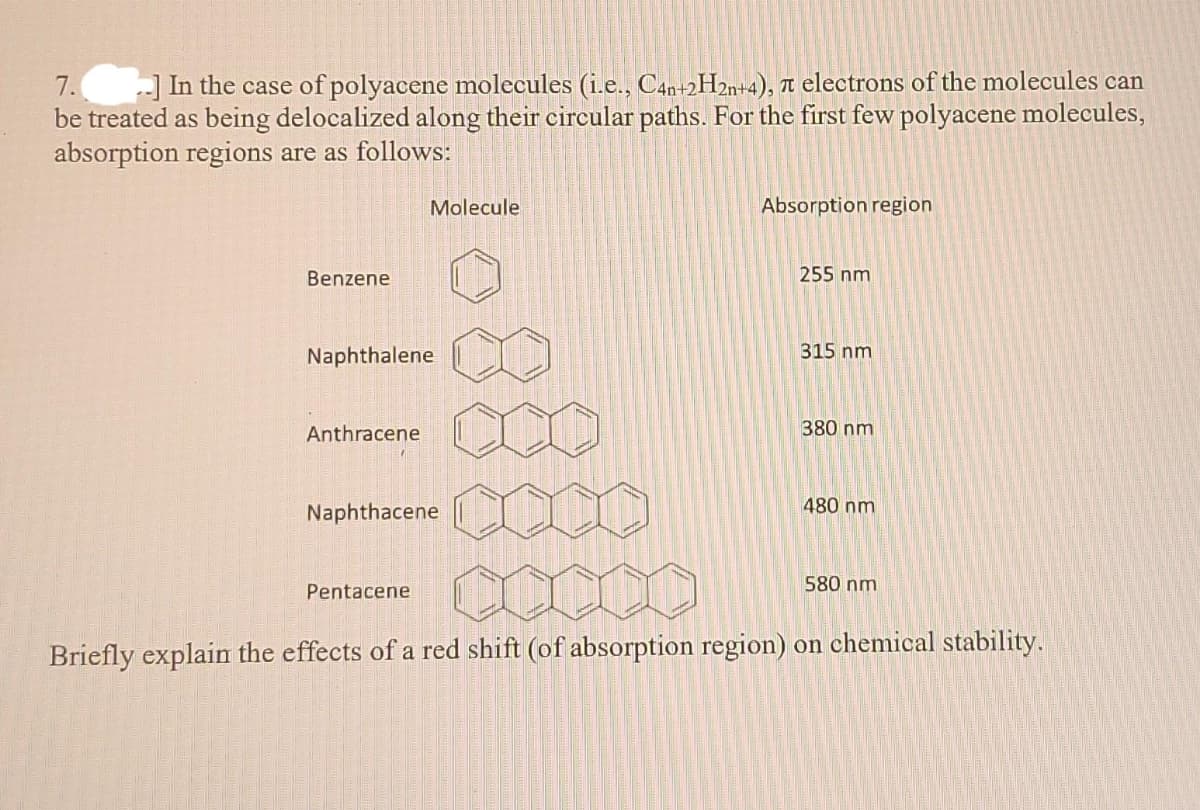
Transcribed Image Text:7.
be treated as being delocalized along their circular paths. For the first few polyacene molecules,
absorption regions are as follows:
) In the case of polyacene molecules (i.e., C4n+2H2%+4), n electrons of the molecules can
Molecule
Absorption region
Benzene
255 nm
Naphthalene
315 nm
Anthracene
380 nm
480 nm
Naphthacene
580 nm
Pentacene
Briefly explain the effects of a red shift (of absorption region) on chemical stability.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning

Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning

Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,


Chemistry for Engineering Students
Chemistry
ISBN:
9781285199023
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning