The following are three MOs for the heptatrienyl cation. For each MO, (a) determine the number of nodal planes perpendicular to the bonding axes and rank them in order of increasing energy; (b) draw the p AO contributions on each C atom that would give rise to the MO; (c) identify each internuclear region as having either a bonding or an antibonding type of contribution; and (d) based on your answer to part (c), determine whether each MO is overall bonding, nonbonding, or antibonding. A B
The following are three MOs for the heptatrienyl cation. For each MO, (a) determine the number of nodal planes perpendicular to the bonding axes and rank them in order of increasing energy; (b) draw the p AO contributions on each C atom that would give rise to the MO; (c) identify each internuclear region as having either a bonding or an antibonding type of contribution; and (d) based on your answer to part (c), determine whether each MO is overall bonding, nonbonding, or antibonding. A B
Principles of Modern Chemistry
8th Edition
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Chapter6: Quantum Mechanics And Molecular Structure
Section: Chapter Questions
Problem 64P: For each of the following molecules, construct the MOs from the 2pz atomic orbitals perpendicular...
Related questions
Question
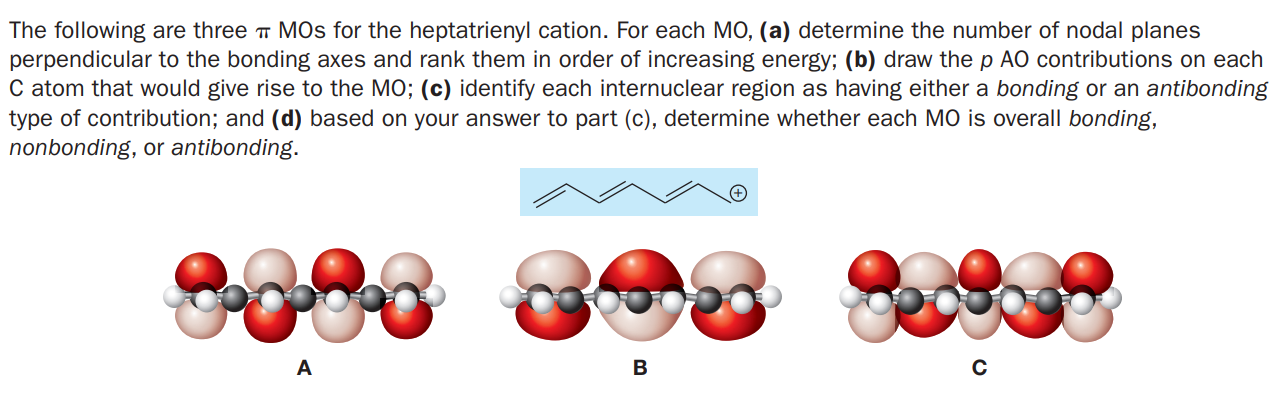
Transcribed Image Text:The following are three MOs for the heptatrienyl cation. For each MO, (a) determine the number of nodal planes
perpendicular to the bonding axes and rank them in order of increasing energy; (b) draw the p AO contributions on each
C atom that would give rise to the MO; (c) identify each internuclear region as having either a bonding or an antibonding
type of contribution; and (d) based on your answer to part (c), determine whether each MO is overall bonding,
nonbonding, or antibonding.
A
B
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 6 images

Recommended textbooks for you

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning