The following initial rate data are for the reaction of ammonium ion with nitrite ion in aqueous solution NH4+ + NO2 → N2 + 2 H2O Experiment 0.776 0.776 1.55 1.55 NO2l, M 4.80x10-2 9.60x10-2 4.80×10-2 9.60x10-2 Initial Rate, Ms1 1.29×10-5 2.58x10-5 2.57x10-5 5.15x10-4 Complete the rate law for this reaction in the box below. Use the form k A m B n where T is understood for m or n and concentrations taken to the zero power do not appear. Don't enter 1 for m 01 In Rate = From these data, the rate constant is 1--1
The following initial rate data are for the reaction of ammonium ion with nitrite ion in aqueous solution NH4+ + NO2 → N2 + 2 H2O Experiment 0.776 0.776 1.55 1.55 NO2l, M 4.80x10-2 9.60x10-2 4.80×10-2 9.60x10-2 Initial Rate, Ms1 1.29×10-5 2.58x10-5 2.57x10-5 5.15x10-4 Complete the rate law for this reaction in the box below. Use the form k A m B n where T is understood for m or n and concentrations taken to the zero power do not appear. Don't enter 1 for m 01 In Rate = From these data, the rate constant is 1--1
Chemistry for Engineering Students
4th Edition
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Lawrence S. Brown, Tom Holme
Chapter11: Chemical Kinetics
Section: Chapter Questions
Problem 11.32PAE: 11.32 The following experimental data were obtained for the reaction 2A + 3 B—C + 2D [A](mol L 1)...
Related questions
Question
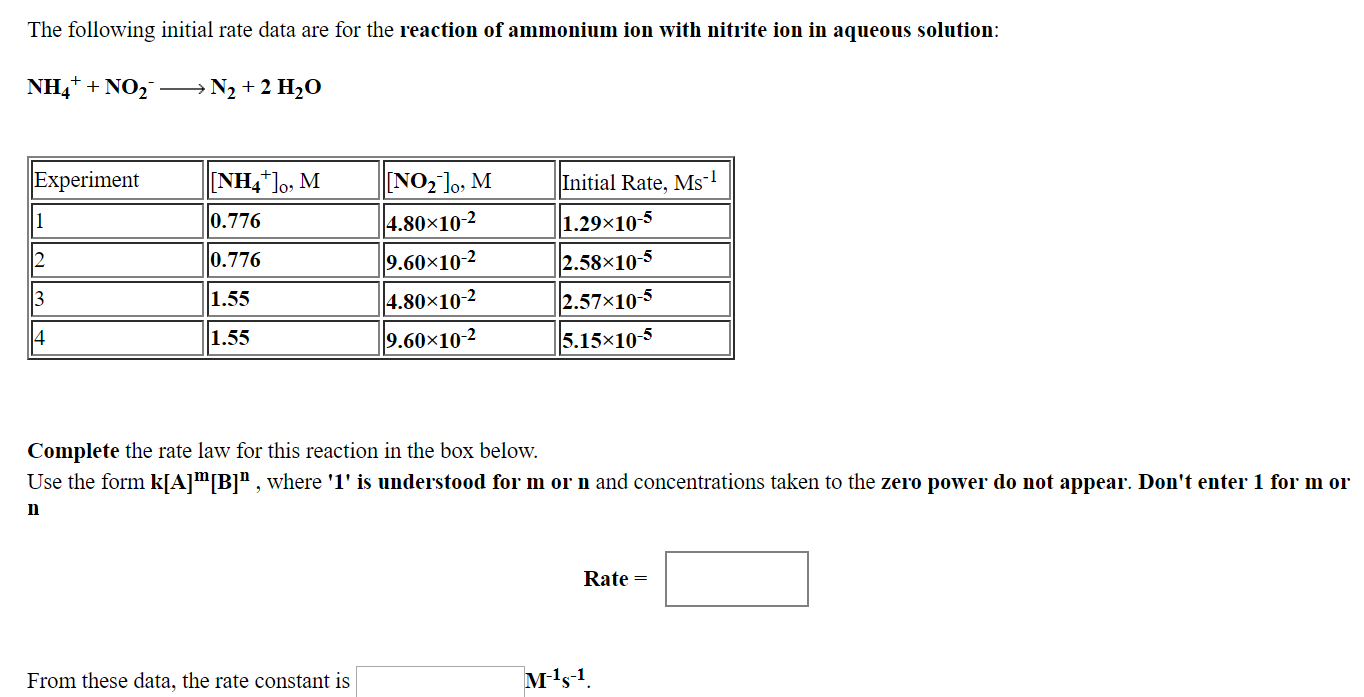
Transcribed Image Text:The following initial rate data are for the reaction of ammonium ion with nitrite ion in aqueous solution
NH4+ + NO2
→ N2 + 2 H2O
Experiment
0.776
0.776
1.55
1.55
NO2l, M
4.80x10-2
9.60x10-2
4.80×10-2
9.60x10-2
Initial Rate, Ms1
1.29×10-5
2.58x10-5
2.57x10-5
5.15x10-4
Complete the rate law for this reaction in the box below.
Use the form k A m B n where T is understood for m or n and concentrations taken to the zero power do not appear. Don't enter 1 for m 01
In
Rate =
From these data, the rate constant is
1--1
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Step 1
VIEWTrending now
This is a popular solution!
Step by step
Solved in 1 steps with 1 images

Recommended textbooks for you

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning