The following reaction schemes have flaws as presenled. CI -CH3 1.HNO,, H,SO, 2. CH,a, AICI, 3. SnCh, H,0* 4. NaOH, H;0 NH2 O A. Step 1 (nitration) of the reaction cannot occur after chlorination O B. After nitration, the alkylation reaction cannot occur OC. The reduction of NO2 by SnCl2,H30* to NH2 should be the last step O D. All reaction steps cannot occur since the ring is deactivated
The following reaction schemes have flaws as presenled. CI -CH3 1.HNO,, H,SO, 2. CH,a, AICI, 3. SnCh, H,0* 4. NaOH, H;0 NH2 O A. Step 1 (nitration) of the reaction cannot occur after chlorination O B. After nitration, the alkylation reaction cannot occur OC. The reduction of NO2 by SnCl2,H30* to NH2 should be the last step O D. All reaction steps cannot occur since the ring is deactivated
Organic Chemistry
8th Edition
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Chapter10: Alcohols
Section: Chapter Questions
Problem 10.50P
Related questions
Question
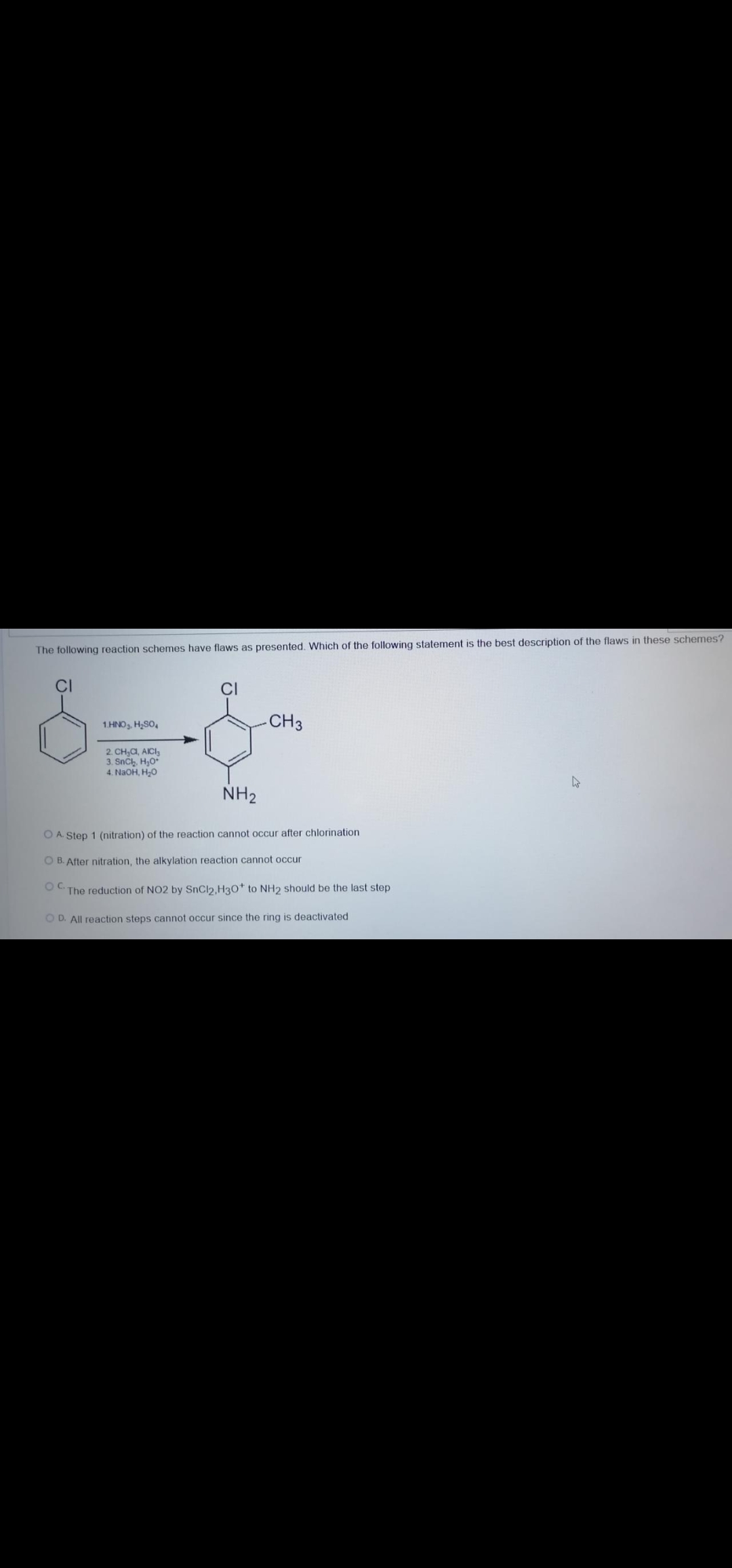
Transcribed Image Text:The following reaction schemes have flaws as presented. Which of the following statement is the best description of the flaws in these schemes?
CI
CI
1.HNO, H,SO,
-CH3
2. CH,C, AICI,
3. Snčh, H,0
4. NaOH, H-0
NH2
O A. Step 1 (nitration) of the reaction cannot occur after chlorination
O B. After nitration, the alkylation reaction cannot occur
Oc.
The reduction of NO2 by SnCl2,H30* to NH2 should be the last step
O D. All reaction steps cannot occur since the ring is deactivated
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning



Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning

