The following table is the data table recorded in the lab for the reactions listed on in your VCL 3-12. Parameter Reaction 1 Reaction 2 Reaction 3 Mass NaOH 3.9982 3.9958 Initial temperature (°C) 25.00 25.00 24.99 30.05 36.05 30.84 Final temperature (°C) Assuming the experiments are done exactly as describe in vCL 3-12, calculated the energy released, kJ/mol, of NaOH in each reaction. Show all calculation steps.
The following table is the data table recorded in the lab for the reactions listed on in your VCL 3-12. Parameter Reaction 1 Reaction 2 Reaction 3 Mass NaOH 3.9982 3.9958 Initial temperature (°C) 25.00 25.00 24.99 30.05 36.05 30.84 Final temperature (°C) Assuming the experiments are done exactly as describe in vCL 3-12, calculated the energy released, kJ/mol, of NaOH in each reaction. Show all calculation steps.
Physical Chemistry
2nd Edition
ISBN:9781133958437
Author:Ball, David W. (david Warren), BAER, Tomas
Publisher:Ball, David W. (david Warren), BAER, Tomas
Chapter5: Introduction To Chemical Equilibrium
Section: Chapter Questions
Problem 5.44E: Biological standard states include specifying a reference temperature of 37.0C rather than the...
Related questions
Question
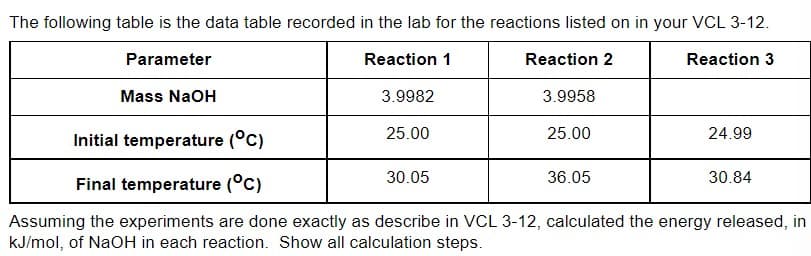
Transcribed Image Text:The following table is the data table recorded in the lab for the reactions listed on in your VCL 3-12.
Parameter
Reaction 1
Reaction 2
Reaction 3
Mass NaOH
3.9982
3.9958
25.00
25.00
24.99
Initial temperature (°C)
30.05
36.05
30.84
Final temperature (°C)
Assuming the experiments are done exactly as describe in VCL 3-12, calculated the energy released, in
kJ/mol, of NaOH in each reaction. Show all calculation steps.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning
