The homogeneous dimerization of butadiene: 2C,H(g) = CgH12(g), has been studied by a number of investigators and found to have an experimental activation energy of 23960 cal/ mol, as indicated by the following equation: %3D k = 9.2 x 10°e-23960/RT cm³mol-'s-1 a. What proportion of the collisions between C4H6 molecules have enough energy to result in reaction when the temperature is 200°C? b. Use the collision theory to predict a value of the pre-exponential factor (in SI units) at 600 K. Assume that the collision diameter is 5 x 10-cm. c. By how many times the calculated value of the pre-exponential factor is greater than the experimental value? Suggest a reason for this discrepancy.
The homogeneous dimerization of butadiene: 2C,H(g) = CgH12(g), has been studied by a number of investigators and found to have an experimental activation energy of 23960 cal/ mol, as indicated by the following equation: %3D k = 9.2 x 10°e-23960/RT cm³mol-'s-1 a. What proportion of the collisions between C4H6 molecules have enough energy to result in reaction when the temperature is 200°C? b. Use the collision theory to predict a value of the pre-exponential factor (in SI units) at 600 K. Assume that the collision diameter is 5 x 10-cm. c. By how many times the calculated value of the pre-exponential factor is greater than the experimental value? Suggest a reason for this discrepancy.
Chemistry & Chemical Reactivity
9th Edition
ISBN:9781133949640
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Chapter14: Chemical Kinetics: The Rates Of Chemical Reactions
Section: Chapter Questions
Problem 86IL: The acid-catalyzed iodination of acetone CH3COCH3(aq) + I2(aq) CH3COCH2I(aq) + HI(aq) is a common...
Related questions
Question
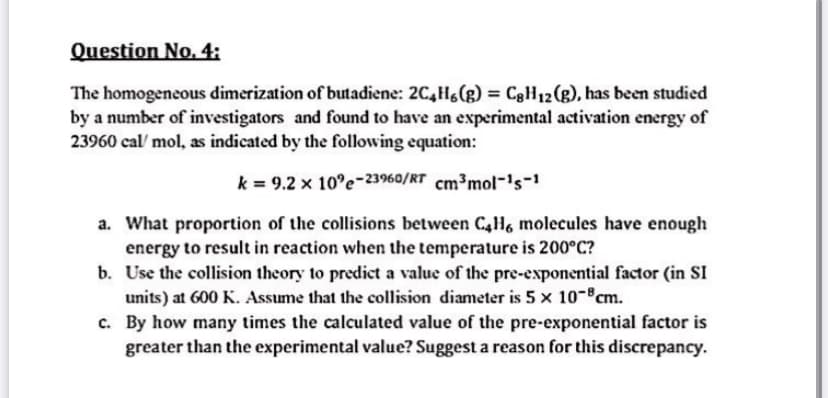
Transcribed Image Text:Question No., 4:
The homogeneous dimerization of butadiene: 2C,Hs(g) = C8H12(g), has been studied
by a number of investigators and found to have an experimental activation energy of
23960 cal/ mol, as indicated by the following equation:
k = 9.2 x 10°e-23960/RT cm³mol-'s-1
a. What proportion of the collisions between C4H6 molecules have enough
energy to result in reaction when the temperature is 200°C?
b. Use the collision theory to predict a value of the pre-exponential factor (in SI
units) at 600 K. Assume that the collision diameter is 5 x 10-°cm.
c. By how many times the calculated value of the pre-exponential factor is
greater than the experimental value? Suggest a reason for this discrepancy.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,
