The most common method for the synthesis of unsymmetrical ethers is the Williamson synthesis, a reaction (SN2) of an alkoxide ion with an alkyl halide. Two pathways are possible, but often one is preferred. Construct the preferred pathway for the synthesis of 2-propoxypropane from propene, with propene-derived alkyl halide and alkoxide intermediates, by dragging the appropriate intermediates and reagents into their bins. Not every given reagent or intermediate will be used. HBr ROOR Но Br Step 1 Product NaH HBr Reagent 1 CH2CI2 H20 H2SO4 propene B2H6 H2O2 Br Reagent 2 Reagent 3 НО Step 2 Product Step 3 Product Na+ Nat - NaBr 2-propoxypropane
The most common method for the synthesis of unsymmetrical ethers is the Williamson synthesis, a reaction (SN2) of an alkoxide ion with an alkyl halide. Two pathways are possible, but often one is preferred. Construct the preferred pathway for the synthesis of 2-propoxypropane from propene, with propene-derived alkyl halide and alkoxide intermediates, by dragging the appropriate intermediates and reagents into their bins. Not every given reagent or intermediate will be used. HBr ROOR Но Br Step 1 Product NaH HBr Reagent 1 CH2CI2 H20 H2SO4 propene B2H6 H2O2 Br Reagent 2 Reagent 3 НО Step 2 Product Step 3 Product Na+ Nat - NaBr 2-propoxypropane
Organic Chemistry
8th Edition
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Chapter9: Nucleophilic Substitution And Β-elimination
Section: Chapter Questions
Problem 9.48P: The Williamson ether synthesis involves treatment of a haloalkane with a metal alkoxide. Following...
Related questions
Question
100%
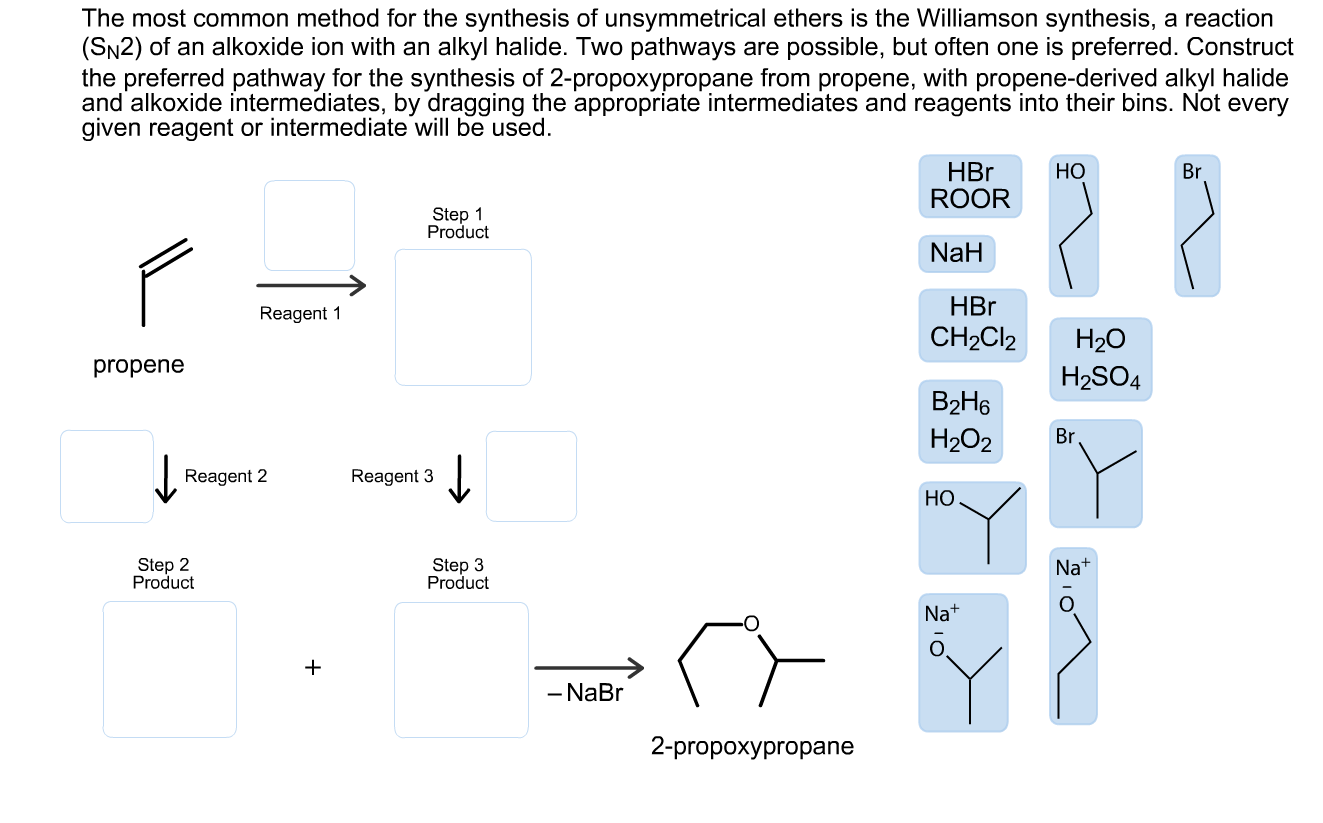
Transcribed Image Text:The most common method for the synthesis of unsymmetrical ethers is the Williamson synthesis, a reaction
(SN2) of an alkoxide ion with an alkyl halide. Two pathways are possible, but often one is preferred. Construct
the preferred pathway for the synthesis of 2-propoxypropane from propene, with propene-derived alkyl halide
and alkoxide intermediates, by dragging the appropriate intermediates and reagents into their bins. Not every
given reagent or intermediate will be used.
HBr
ROOR
Но
Br
Step 1
Product
NaH
HBr
Reagent 1
CH2CI2
H20
H2SO4
propene
B2H6
H2O2
Br
Reagent 2
Reagent 3
НО
Step 2
Product
Step 3
Product
Na+
Nat
- NaBr
2-propoxypropane
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 5 steps with 3 images

Recommended textbooks for you

Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning

Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning