The naphthalene molecule has a structure that corre- sponds to two benzene molecules fused together: The ™ electrons in this molecule are delocalized over the entire molecule. The wavelength of maximum absorption in benzene is 255 nm. Will the corresponding wavelength in naphthalene be shorter or longer than 255 nm?
The naphthalene molecule has a structure that corre- sponds to two benzene molecules fused together: The ™ electrons in this molecule are delocalized over the entire molecule. The wavelength of maximum absorption in benzene is 255 nm. Will the corresponding wavelength in naphthalene be shorter or longer than 255 nm?
Principles of Modern Chemistry
8th Edition
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Chapter20: Molecular Spectroscopy And Photochemistry
Section: Chapter Questions
Problem 40P
Related questions
Question
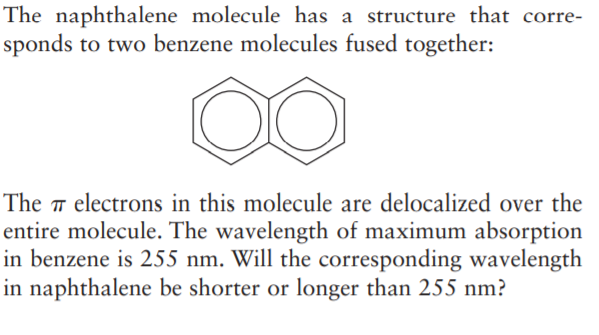
Transcribed Image Text:The naphthalene molecule has a structure that corre-
sponds to two benzene molecules fused together:
The ™ electrons in this molecule are delocalized over the
entire molecule. The wavelength of maximum absorption
in benzene is 255 nm. Will the corresponding wavelength
in naphthalene be shorter or longer than 255 nm?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Recommended textbooks for you

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning