The partial molar volumes of water and ethanol in a solution with H₂O=0.45 at 25 °C are 17.0 and 57.5 cm³ mol-1, respectively. Part A Calculate the volume change upon mixing sufficient ethanol with 6.17 mol of water to give this concentration. The densities of water and ethanol are 0.997 and 0.7893 g cm3, respectively, at this temperature. Express your answer as an integer and include the appropriate units. AV= μA Value Review | Constants | Periodic Table Units
The partial molar volumes of water and ethanol in a solution with H₂O=0.45 at 25 °C are 17.0 and 57.5 cm³ mol-1, respectively. Part A Calculate the volume change upon mixing sufficient ethanol with 6.17 mol of water to give this concentration. The densities of water and ethanol are 0.997 and 0.7893 g cm3, respectively, at this temperature. Express your answer as an integer and include the appropriate units. AV= μA Value Review | Constants | Periodic Table Units
Chapter7: Solutions And Colloids
Section: Chapter Questions
Problem 7.86E
Related questions
Question
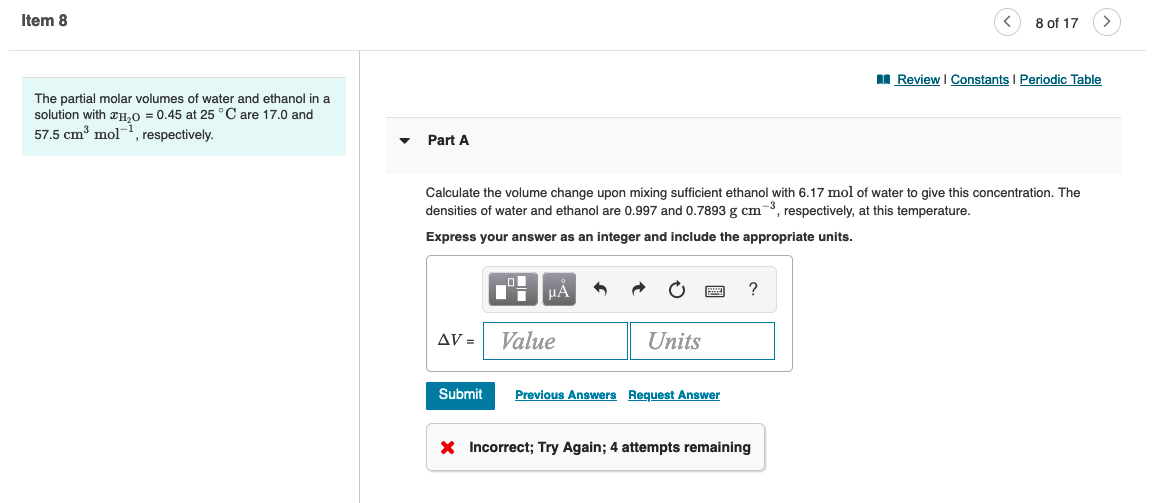
Transcribed Image Text:Item 8
The partial molar volumes of water and ethanol in a
solution with H₂O = 0.45 at 25 °C are 17.0 and
57.5 cm³ mol, respectively.
Part A
AV =
Calculate the volume change upon mixing sufficient ethanol with 6.17 mol of water to give this concentration. The
densities of water and ethanol are 0.997 and 0.7893 g cm³, respectively, at this temperature.
Express your answer as an integer and include the appropriate units.
Submit
μA
Value
Units
Previous Answers Request Answer
?
8 of 17
X Incorrect; Try Again; 4 attempts remaining
Review | Constants | Periodic Table
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning