The peptide aspartyl-glutamyl-leacyl-threony-alanine, shown in the sketch drewing window, has several ionizable proups. Adjust the charges to show the molecule ast would etist at pH 5.00. Use the pk, values in the table below.
The peptide aspartyl-glutamyl-leacyl-threony-alanine, shown in the sketch drewing window, has several ionizable proups. Adjust the charges to show the molecule ast would etist at pH 5.00. Use the pk, values in the table below.
Biochemistry
9th Edition
ISBN:9781319114671
Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Chapter1: Biochemistry: An Evolving Science
Section: Chapter Questions
Problem 1P
Related questions
Question
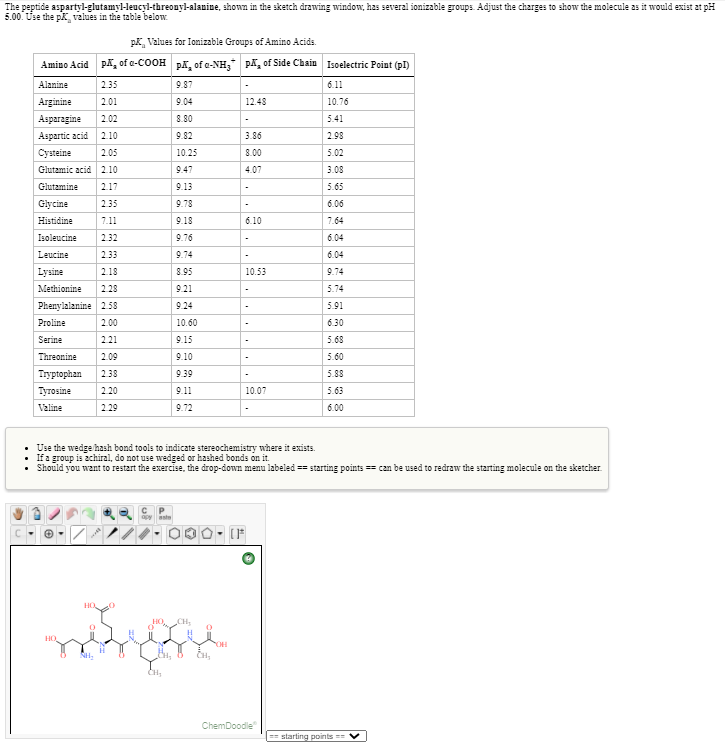
Transcribed Image Text:The peptide aspartyl-glutamyl-leucyl-threonyl-alanine, shown in the sketch drawing window, has several ionizable groups. Adjust the charges to show the molecule as it would exist at pH
5.00. Use the pk values in the table below.
pK, Values for Ionizable Groups of Amino Acids.
Amino Acid pA, of a-COOH pA, of e-NH,
pA, of Side Chain Isoelectric Point (pI)
Alanine
235
9.87
6.11
Arginine
2.01
9.04
12.48
10.76
Asparagine
2.02
8.80
5.41
Aspartic acid
210
9.82
3.86
298
Cysteine
2.05
10.25
8.00
5.02
Glutzmic acid 2.10
9.47
4.07
3.08
Glutamine
217
9.13
5.65
Glycine
235
9.78
6.06
Histidine
7.11
9.18
6.10
7.64
Isoleucine
232
9.76
6.04
Leucine
233
9.74
6.04
Lysine
218
8.95
10.53
9.74
Methionine
2 28
9.21
5.74
Phenylalanine 2 58
9.24
5.91
Proline
2.00
10.60
6.30
Serine
221
9.15
5.68
Threonine
2.09
9.10
5.60
Trуptophan
238
9.39
5.88
Tyrosine
2 20
9.11
10.07
5.63
Valine
2 29
9.72
6.00
• Use the wedge hash bond tools to indicate stereochemistry where it exists.
• If a group is 2chiral, do not use wedged or hashed bonds on it.
Should you want to restart the exercise, the drop-down menu labeled == starting points == can be used to redraw the starting molecule on the sketcher.
apy te
CH
HO
NH,
CH,
CH,
ChemDoodle"
starting points ==
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, biochemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Biochemistry
Biochemistry
ISBN:
9781319114671
Author:
Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher:
W. H. Freeman

Lehninger Principles of Biochemistry
Biochemistry
ISBN:
9781464126116
Author:
David L. Nelson, Michael M. Cox
Publisher:
W. H. Freeman

Fundamentals of Biochemistry: Life at the Molecul…
Biochemistry
ISBN:
9781118918401
Author:
Donald Voet, Judith G. Voet, Charlotte W. Pratt
Publisher:
WILEY

Biochemistry
Biochemistry
ISBN:
9781319114671
Author:
Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher:
W. H. Freeman

Lehninger Principles of Biochemistry
Biochemistry
ISBN:
9781464126116
Author:
David L. Nelson, Michael M. Cox
Publisher:
W. H. Freeman

Fundamentals of Biochemistry: Life at the Molecul…
Biochemistry
ISBN:
9781118918401
Author:
Donald Voet, Judith G. Voet, Charlotte W. Pratt
Publisher:
WILEY

Biochemistry
Biochemistry
ISBN:
9781305961135
Author:
Mary K. Campbell, Shawn O. Farrell, Owen M. McDougal
Publisher:
Cengage Learning

Biochemistry
Biochemistry
ISBN:
9781305577206
Author:
Reginald H. Garrett, Charles M. Grisham
Publisher:
Cengage Learning

Fundamentals of General, Organic, and Biological …
Biochemistry
ISBN:
9780134015187
Author:
John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. Peterson
Publisher:
PEARSON