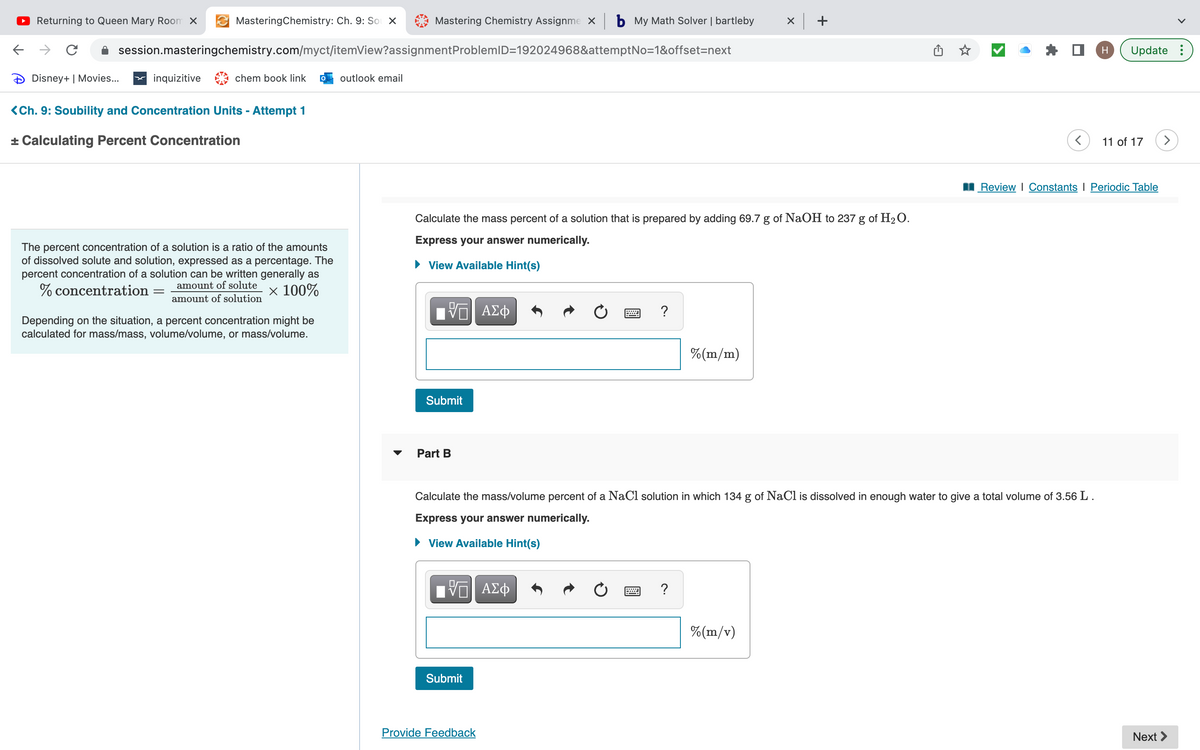
The percent concentration of a solution is a ratio of the amounts of dissolved solute and solution, expressed as a percentage. The percent concentration of a solution can be written generally as % concentration amount of solute amount of solution × 100% Depending on the situation, a percent concentration might be calculated for mass/mass, volume/volume, or mass/volume. Calculate the mass percent of a solution that is prepared by adding 69.7 g of NaOH to 237 g of H₂O. Express your answer numerically. View Available Hint(s) 15| ΑΣΦ Submit Part B VG| ΑΣΦ 3 Submit C ? Calculate the mass/volume percent of a NaCl solution in which 134 g of NaCl dissolved in enough water to give a total volume of 3.56 L. Express your answer numerically. ▸ View Available Hint(s) % (m/m) ? Review | Constants I Periodic Table %(m/v)
The percent concentration of a solution is a ratio of the amounts of dissolved solute and solution, expressed as a percentage. The percent concentration of a solution can be written generally as % concentration amount of solute amount of solution × 100% Depending on the situation, a percent concentration might be calculated for mass/mass, volume/volume, or mass/volume. Calculate the mass percent of a solution that is prepared by adding 69.7 g of NaOH to 237 g of H₂O. Express your answer numerically. View Available Hint(s) 15| ΑΣΦ Submit Part B VG| ΑΣΦ 3 Submit C ? Calculate the mass/volume percent of a NaCl solution in which 134 g of NaCl dissolved in enough water to give a total volume of 3.56 L. Express your answer numerically. ▸ View Available Hint(s) % (m/m) ? Review | Constants I Periodic Table %(m/v)
Chemistry: An Atoms First Approach
2nd Edition
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Steven S. Zumdahl, Susan A. Zumdahl
Chapter16: Spontaneity, Entropy, And Free Energy
Section: Chapter Questions
Problem 107CP: Using data from Appendix 4, calculate H, G, and K (at 298 K) for the production of ozone from...
Related questions
Question
Transcribed Image Text:←
Returning to Queen Mary Room X
с
MasteringChemistry: Ch. 9: Sol X
session.masteringchemistry.com/myct/itemView?assignment
Disney+ | Movies...
inquizitive
chem book link
<Ch. 9: Soubility and Concentration Units - Attempt 1
+ Calculating Percent Concentration
The percent concentration of a solution is a ratio of the amounts
of dissolved solute and solution, expressed as a percentage. The
percent concentration of a solution can be written generally as
% concentration
amount of solute
=
x 100%
amount of solution
Depending on the situation, a percent concentration might be
calculated for mass/mass, volume/volume, or mass/volume.
Mastering Chemistry Assignme × b My Math Solver | bartleby
ProblemID=192024968&attemptNo=1&offset=next
outlook email
IVF ΑΣΦ
Calculate the mass percent of a solution that is prepared by adding 69.7 g of NaOH to 237 g of H₂O.
Express your answer numerically.
► View Available Hint(s)
Submit
Part B
Ε| ΑΣΦ
Submit
....
Provide Feedback
?
% (m/m)
X
Calculate the mass/volume percent of a NaCl solution in which 134 g of NaCl is dissolved in enough water to give a total volume of 3.56 L.
Express your answer numerically.
View Available Hint(s)
+
%(m/v)
H
Update:
11 of 17
Review | Constants | Periodic Table
Next >
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,