The van der Waals equation of state was designed to predict the relabionship between pressure p, volume l'and temperature 7 for gases better than the Ideal Gas Law The van der Waals equation of state, R stands for the gas constant and n for the moles of gas The van der Waals parameters a and b have to be determined for each gas by comparing to experimental data Consider the hypothetical gases X, Y and Z listed in the table below. Use the information provided to decide the relative size of the van der Waals parameters a and h
The van der Waals equation of state was designed to predict the relabionship between pressure p, volume l'and temperature 7 for gases better than the Ideal Gas Law The van der Waals equation of state, R stands for the gas constant and n for the moles of gas The van der Waals parameters a and b have to be determined for each gas by comparing to experimental data Consider the hypothetical gases X, Y and Z listed in the table below. Use the information provided to decide the relative size of the van der Waals parameters a and h
General Chemistry - Standalone book (MindTap Course List)
11th Edition
ISBN:9781305580343
Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Chapter5: The Gaseous State
Section: Chapter Questions
Problem 5.103QP: Calculate the molar volume of ethane at 1.00 atm and 0C and at 10.0 atm and 0C, using the van der...
Related questions
Question
2
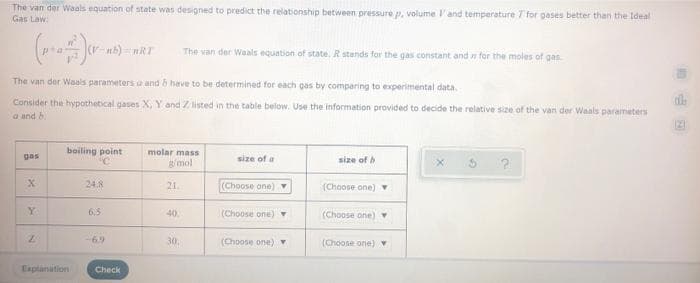
Transcribed Image Text:The van der Waals equation of state was designed to predict the relationship between pressure p, volume l'and temperature T for gases better than the Ideal
Gas Law:
The van der Waals equation of state. R stands for the gas constant and for the moles of gas.
The van der Waals parameters a and h have to be determined for each gas by comparing to experimental data.
Consider the hypothetical gases X, Y and Z listed in the table below. Use the information provided to decide the relative size of the van der Waals parameters
a and h
boiling point
molar mass
g/mol
gas
size of a
size of
24.8
21
(Choose one) Y
(Choose one) v
Y.
6.5
(Choose one)
40
(Choose one)
6.9
30.
(Choose one) v
(Choone one) Y
Explanation
Check
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 5 steps

Recommended textbooks for you

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co