The prevailing atmospheric pressure on a plateau in Colorado is 0.770 atm. Express this pressure in pounds per square inch, kilopascals, pascals, millimeters Hg, and inches Hg. Hint: 1 atm = 101.3 kPa = 1.013×10° Pa = 760 mm Hg = 14.69 psi = 29.92 in Hg atm psi kPa Pa mm Hg in Hg 0.770
The prevailing atmospheric pressure on a plateau in Colorado is 0.770 atm. Express this pressure in pounds per square inch, kilopascals, pascals, millimeters Hg, and inches Hg. Hint: 1 atm = 101.3 kPa = 1.013×10° Pa = 760 mm Hg = 14.69 psi = 29.92 in Hg atm psi kPa Pa mm Hg in Hg 0.770
Chemistry & Chemical Reactivity
10th Edition
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Chapter10: Gases And Their Properties
Section10.2: Gas Laws: The Experimental Basis
Problem 10.4CYU: You have a 22-L cylinder of helium at a pressure of 150 atm (above atmospheric pressure) and at 31...
Related questions
Question
100%
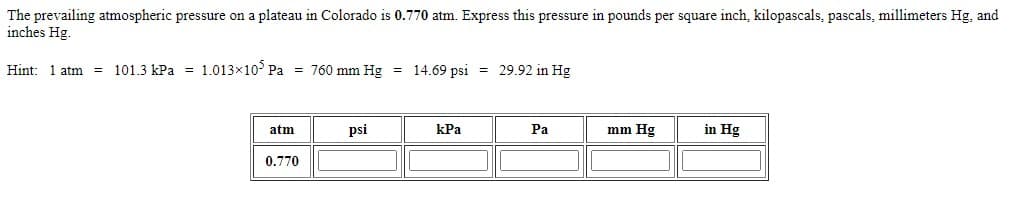
Transcribed Image Text:The prevailing atmospheric pressure on a plateau in Colorado is 0.770 atm. Express this pressure in pounds per square inch, kilopascals, pascals, millimeters Hg, and
inches Hg.
Hint: 1 atm = 101.3 kPa = 1.013x10° Pa = 760 mm Hg = 14.69 psi = 29.92 in Hg
atm
psi
kPa
Pa
mm Hg
in Hg
0.770
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Recommended textbooks for you

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning