The questions below refer to the following system: Co(H,O)6+4 CI =C0C14 + 6H,O (pink) (blue) When cobalt(II) chloride is added to pure water, the Co" ions hydrate. The hydrated form then reacts with the Cl ions to set up the equilibrium shown here. 1S Which statement below describes the change that the system will undergo if water is added? Select one: O a. More chloride ions will be produced. O b. The equilibrium will shift to the right. O c. There will be less of the hydrated cobalt ion at the new equilibrium position. O d. More water will be produced. O e. The color will become more blue.
The questions below refer to the following system: Co(H,O)6+4 CI =C0C14 + 6H,O (pink) (blue) When cobalt(II) chloride is added to pure water, the Co" ions hydrate. The hydrated form then reacts with the Cl ions to set up the equilibrium shown here. 1S Which statement below describes the change that the system will undergo if water is added? Select one: O a. More chloride ions will be produced. O b. The equilibrium will shift to the right. O c. There will be less of the hydrated cobalt ion at the new equilibrium position. O d. More water will be produced. O e. The color will become more blue.
Chemistry: Principles and Practice
3rd Edition
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Chapter14: Chemical Equilibrium
Section: Chapter Questions
Problem 14.86QE
Related questions
Question
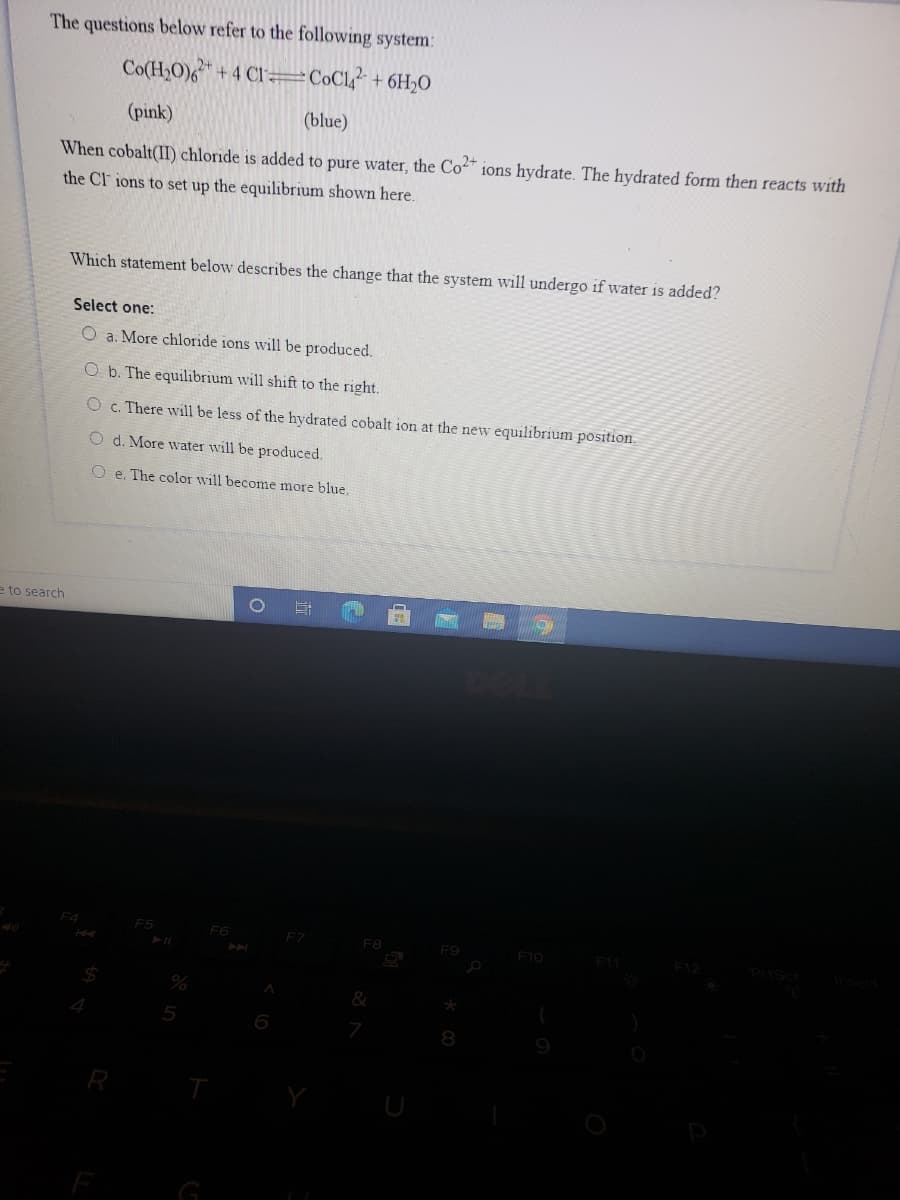
Transcribed Image Text:The questions below refer to the following system:
Co(H,O)6+4 CI =C©C1? + 6H20
(pink)
(blue)
When cobalt(II) chloride is added to pure water, the Co" ions hydrate. The hydrated form then reacts with
the Cl ions to set up the equilibrium shown here.
Which statement below describes the change that the system will undergo if water is added?
Select one:
O a. More chloride ions will be produced.
O b. The equilibrium will shift to the right.
O c. There will be less of the hydrated cobalt ion at the new equilibrium position.
O d. More water will be produced.
O e. The color will become more blue.
e to search
F4
F5
F7
F8
F9
F10
7
8
9
Expert Solution
Step 1
Le Chatelier's principle:
This principle is used to predict the effect of a change in conditions on chemical equilibrium. This principle states that when any system at equilibrium for a long period of time is subjected to a change in concentration, temperature, volume, or pressure the system changes to a new equilibrium, and this change partly counteracts the applied change.
Trending now
This is a popular solution!
Step by step
Solved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning