The rate constant for a reaction is 1.47 x 104 L mol¹ s1 at 303 K and 9.31 x 10-3 L mol-1 s 1 at 330 K. Calculate the activation energy (E₂) for this reaction (R= 8.3145 J K¹¹ mol¹). 128 kJ mol-1 -199 kJ mol-1 199 kJ mol-1 -128 kJ mol-1 Show work, don't give Handwritten answer
The rate constant for a reaction is 1.47 x 104 L mol¹ s1 at 303 K and 9.31 x 10-3 L mol-1 s 1 at 330 K. Calculate the activation energy (E₂) for this reaction (R= 8.3145 J K¹¹ mol¹). 128 kJ mol-1 -199 kJ mol-1 199 kJ mol-1 -128 kJ mol-1 Show work, don't give Handwritten answer
Chemistry for Engineering Students
4th Edition
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Lawrence S. Brown, Tom Holme
Chapter11: Chemical Kinetics
Section: Chapter Questions
Problem 11.45PAE: The initial concentration of the reactant in a tirst-order reaction A —» products is 0.64 rnol/L and...
Related questions
Question
None
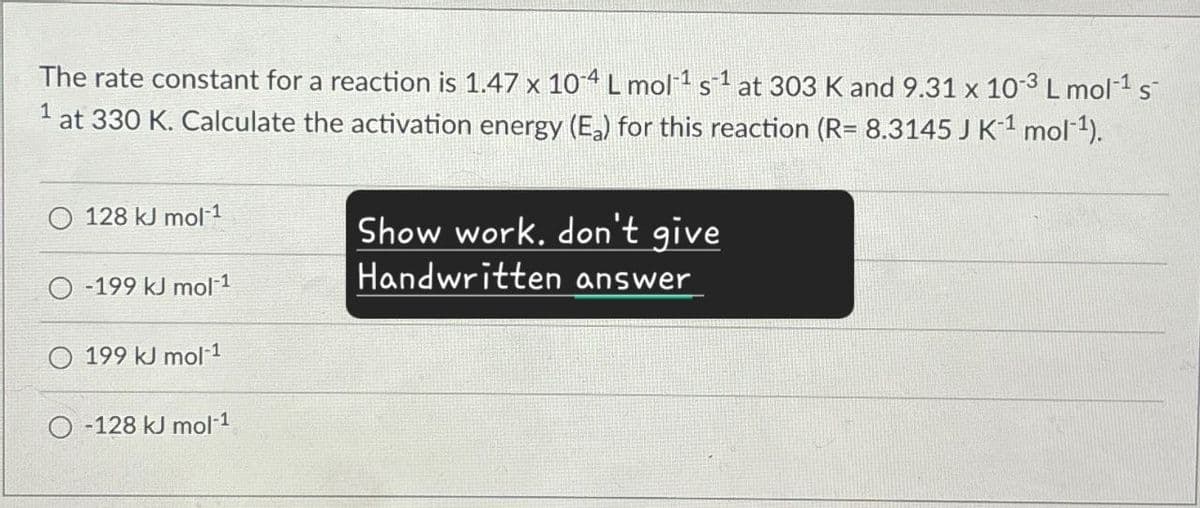
Transcribed Image Text:The rate constant for a reaction is 1.47 x 104 L mol¹ s1 at 303 K and 9.31 x 10-3 L mol-1 s
1 at 330 K. Calculate the activation energy (E₂) for this reaction (R= 8.3145 J K¹¹ mol¹).
128 kJ mol-1
-199 kJ mol-1
199 kJ mol-1
-128 kJ mol-1
Show work, don't give
Handwritten answer
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps

Recommended textbooks for you

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning