The rate of the electron-exchange reaction between naphthalene (C10H8) and its anion radical (C10H8¬) in an organic solvent is diffusion controlled. C10H8¯ + C10H8=C10H8 + C10H8¯ The reaction is bimolecular and second order. The rate constants are as follows. T/K 305 295 291 275 k/109 M-1.s-1 2.64 2.21 2.05 1.49 Calculate the values of Ea, AHo#, ASº† and AGº† at 295 K for the reaction. [Hint: Rearrange the equation below and plot In(k/T) versus 1/T.] k = h kBT Asot/Re-AH®?/RT(M1 – m)
The rate of the electron-exchange reaction between naphthalene (C10H8) and its anion radical (C10H8¬) in an organic solvent is diffusion controlled. C10H8¯ + C10H8=C10H8 + C10H8¯ The reaction is bimolecular and second order. The rate constants are as follows. T/K 305 295 291 275 k/109 M-1.s-1 2.64 2.21 2.05 1.49 Calculate the values of Ea, AHo#, ASº† and AGº† at 295 K for the reaction. [Hint: Rearrange the equation below and plot In(k/T) versus 1/T.] k = h kBT Asot/Re-AH®?/RT(M1 – m)
Chemistry: The Molecular Science
5th Edition
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:John W. Moore, Conrad L. Stanitski
Chapter11: Chemical Kinetics: Rates Of Reactions
Section11.3: Rate Law And Order Of Reactions
Problem 11.5PSP
Related questions
Question
100%
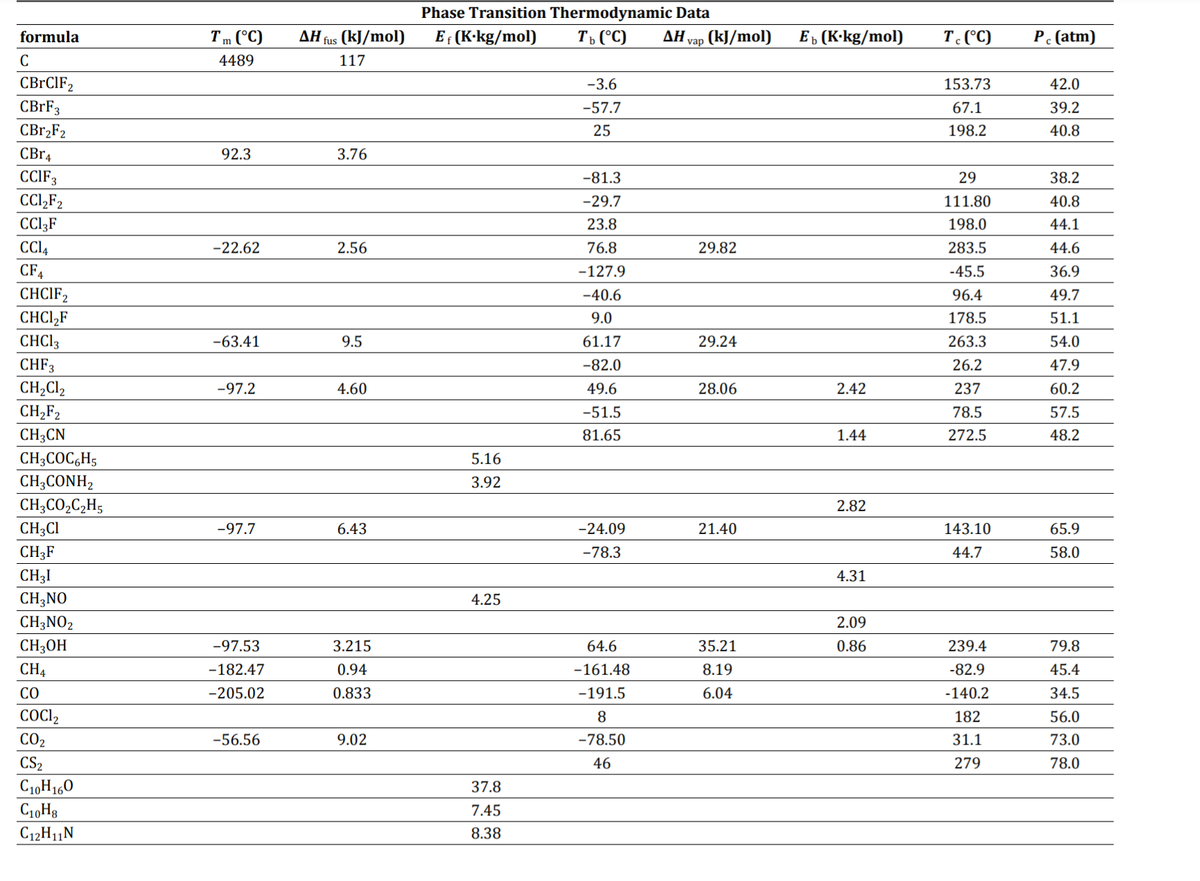
Transcribed Image Text:Phase Transition Thermodynamic Data
formula
Tm (°C)
AH fus (kJ/mol)
E (K-kg/mol)
T, (°C)
ΔΗ
vap (kJ/mol)
Еъ (К-kg/mol)
T. (°C)
P (atm)
C
4489
117
CBrCIF2
-3.6
153.73
42.0
CBRF3
CB12F2
CBr4
-57.7
67.1
39.2
25
198.2
40.8
92.3
3.76
CCIF3
-81.3
29
38.2
CL,F2
CCI3F
-29.7
111.80
40.8
23.8
198.0
44.1
CC14
-22.62
2.56
76.8
29.82
283.5
44.6
CF4
CHCIF2
-127.9
-45.5
36.9
-40.6
96.4
49.7
CHCI,F
CHC13
9.0
178.5
51.1
-63.41
9.5
61.17
29.24
263.3
54.0
CHF3
-82.0
26.2
47.9
CH,Cl,
CH2F2
CH3CN
CH;COC,H5
CH3CONH,
CH;CO,C,H5
-97.2
4.60
49.6
28.06
2.42
237
60.2
-51.5
78.5
57.5
81.65
1.44
272.5
48.2
5.16
3.92
2.82
CH3CI
-97.7
6.43
-24.09
21.40
143.10
65.9
CH3F
-78.3
44.7
58.0
CH3I
4.31
CH;NO
4.25
CH;NO2
2.09
CH3OH
-97.53
3.215
64.6
35.21
0.86
239.4
79.8
CH4
-182.47
0.94
-161.48
8.19
-82.9
45.4
CO
-205.02
0.833
-191.5
6.04
-140.2
34.5
COCI,
8
182
56.0
CO2
-56.56
9.02
-78.50
31.1
73.0
CS2
46
279
78.0
C19H160
C10H8
C12H11N
37.8
7.45
8.38
![Please use the values in the resources listed below instead of the textbook values.
The rate of the electron-exchange reaction between naphthalene (C10H8) and its anion radical (C10H8¯) in an organic solvent is diffusion controlled.
C10H8 + C10H8=C10H8 + C10H8-
The reaction is bimolecular and second order. The rate constants are as follows.
T/K
305
295
291
275
k/109 M-1.s-1 2.64 2.21 2.05 1.49
Calculate the values of Ea, AH°†, AS°† and AG°† at 295 K for the reaction. [Hint: Rearrange the equation below and plot In(k/T) versus 1/T.]
kBT ASot/Re-AH°ł/RT(M1 – m)
k =
h
Ea =
17.8
X kJ•mol-1
AH°# =
12.9
X kJ•mol-1
ASot = |-22.3
X J-K-1.mol-1
AG°# =
19.5
kJ•mol-1](/v2/_next/image?url=https%3A%2F%2Fcontent.bartleby.com%2Fqna-images%2Fquestion%2F14e51fbd-8bc7-4604-ba1b-5c934c5c4cfb%2F65c2c883-6646-4844-acc3-675503235ea0%2Fc3x51e_processed.png&w=3840&q=75)
Transcribed Image Text:Please use the values in the resources listed below instead of the textbook values.
The rate of the electron-exchange reaction between naphthalene (C10H8) and its anion radical (C10H8¯) in an organic solvent is diffusion controlled.
C10H8 + C10H8=C10H8 + C10H8-
The reaction is bimolecular and second order. The rate constants are as follows.
T/K
305
295
291
275
k/109 M-1.s-1 2.64 2.21 2.05 1.49
Calculate the values of Ea, AH°†, AS°† and AG°† at 295 K for the reaction. [Hint: Rearrange the equation below and plot In(k/T) versus 1/T.]
kBT ASot/Re-AH°ł/RT(M1 – m)
k =
h
Ea =
17.8
X kJ•mol-1
AH°# =
12.9
X kJ•mol-1
ASot = |-22.3
X J-K-1.mol-1
AG°# =
19.5
kJ•mol-1
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 7 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning


Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning
