The reactant shown, having an axial p-toluenesulfonate in its most stable conformation, undergoes predominant elimination on reaction with sodium azide. TsO NaN3 N3 (46%) (37%) Its diastereomer having an equatorial p-toluenesulfonate gives predominant substitution (76%). Give the structure of the resulting azide and suggest an explanation for the difference in reactivity between the two diastereomeric p-toluenesulfonates.
The reactant shown, having an axial p-toluenesulfonate in its most stable conformation, undergoes predominant elimination on reaction with sodium azide. TsO NaN3 N3 (46%) (37%) Its diastereomer having an equatorial p-toluenesulfonate gives predominant substitution (76%). Give the structure of the resulting azide and suggest an explanation for the difference in reactivity between the two diastereomeric p-toluenesulfonates.
Organic Chemistry
8th Edition
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Chapter21: Benzene And The Concept Of Aromaticity
Section: Chapter Questions
Problem 21.55P
Related questions
Concept explainers
Organomagnesium compounds
The interaction of alkyl halide with Mg metal in a suitable ether solvent is the common method for the synthesis of the Grignard reagent.
Hydrolysis Grignard Reactions and Reduction
Organomagnesium halides are Grignard reagents. Francois Auguste Victor Grignard, a French chemist who received the Nobel Prize in Chemistry in 1912, created these highly useful reagents.
Question
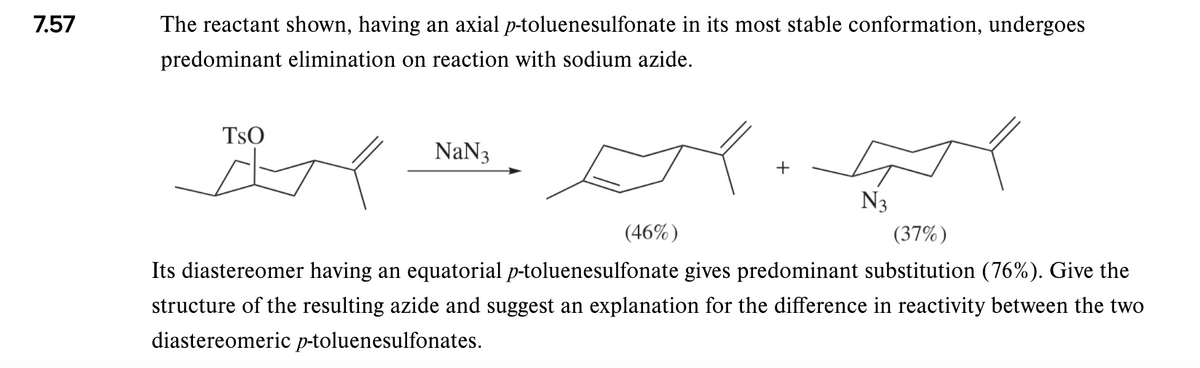
Transcribed Image Text:7.57
The reactant shown, having an axial p-toluenesulfonate in its most stable conformation, undergoes
predominant elimination on reaction with sodium azide.
TsO
NaN3
+
N3
(46%)
(37%)
Its diastereomer having an equatorial p-toluenesulfonate gives predominant substitution (76%). Give the
structure of the resulting azide and suggest an explanation for the difference in reactivity between the two
diastereomeric p-toluenesulfonates.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning


Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning
