The reaction that was on the screen when you started and its derivative demonstrate that the reaction enthalpy, ΔH, changes sign when a process is reversed. Consider the reaction H2O(l)→H2O(g), ΔH =44.0kJ What will ΔH be for the reaction if it is reversed?
The reaction that was on the screen when you started and its derivative demonstrate that the reaction enthalpy, ΔH, changes sign when a process is reversed. Consider the reaction H2O(l)→H2O(g), ΔH =44.0kJ What will ΔH be for the reaction if it is reversed?
Chemistry: Principles and Reactions
8th Edition
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:William L. Masterton, Cecile N. Hurley
Chapter8: Thermochemistry
Section: Chapter Questions
Problem 17QAP: Acetic acid, HC2H3O2, is responsible for the sour taste of vinegar. Combustion of acetic acid gives...
Related questions
Question
The reaction that was on the screen when you started and its derivative demonstrate that the reaction enthalpy, ΔH, changes sign when a process is reversed.
Consider the reaction
H2O(l)→H2O(g), ΔH =44.0kJ
What will ΔH be for the reaction if it is reversed?
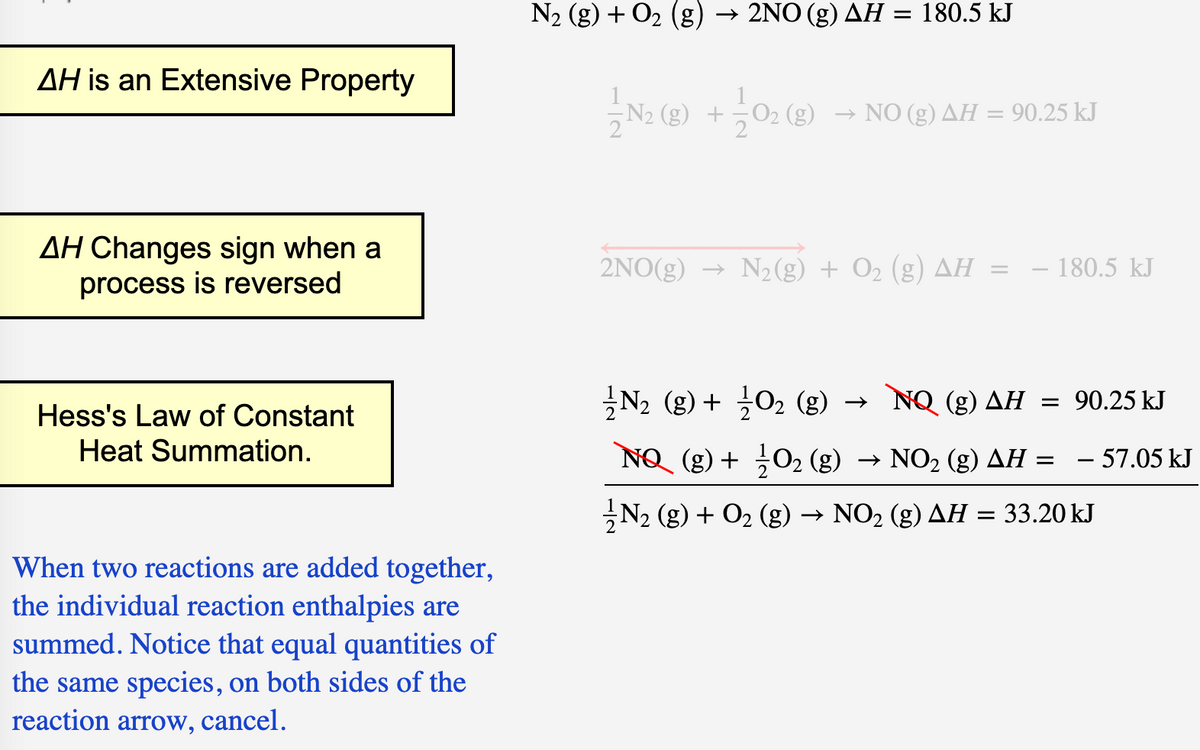
Transcribed Image Text:N2 (g) + O2 (g) → 2NO (g) AH = 180.5 kJ
AH is an Extensive Property
(g)
NO (g) AH = 90.25 kJ
AH Changes sign when a
process is reversed
2NO(g) → N2(g) + O2 (g) AH
- 180.5 kJ
글N2 (g) + 글02 (g) →
NQ (g) AH = 90.25 kJ
Hess's Law of Constant
Heat Summation.
NO (g) + 02 (g) → NO2 (g) AH = - 57.05 kJ
N2 (g) + O2 (g) → NO2 (g) AH = 33.20 kJ
When two reactions are added together,
the individual reaction enthalpies are
summed. Notice that equal quantities of
the same species, on both sides of the
reaction arrow, cancel.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning