Chemistry
10th Edition
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Chapter1: Chemical Foundations
Section: Chapter Questions
Problem 101AE: This year, like many past years, you begin to feel very sleepy alter eating a large helping of...
Related questions
Question
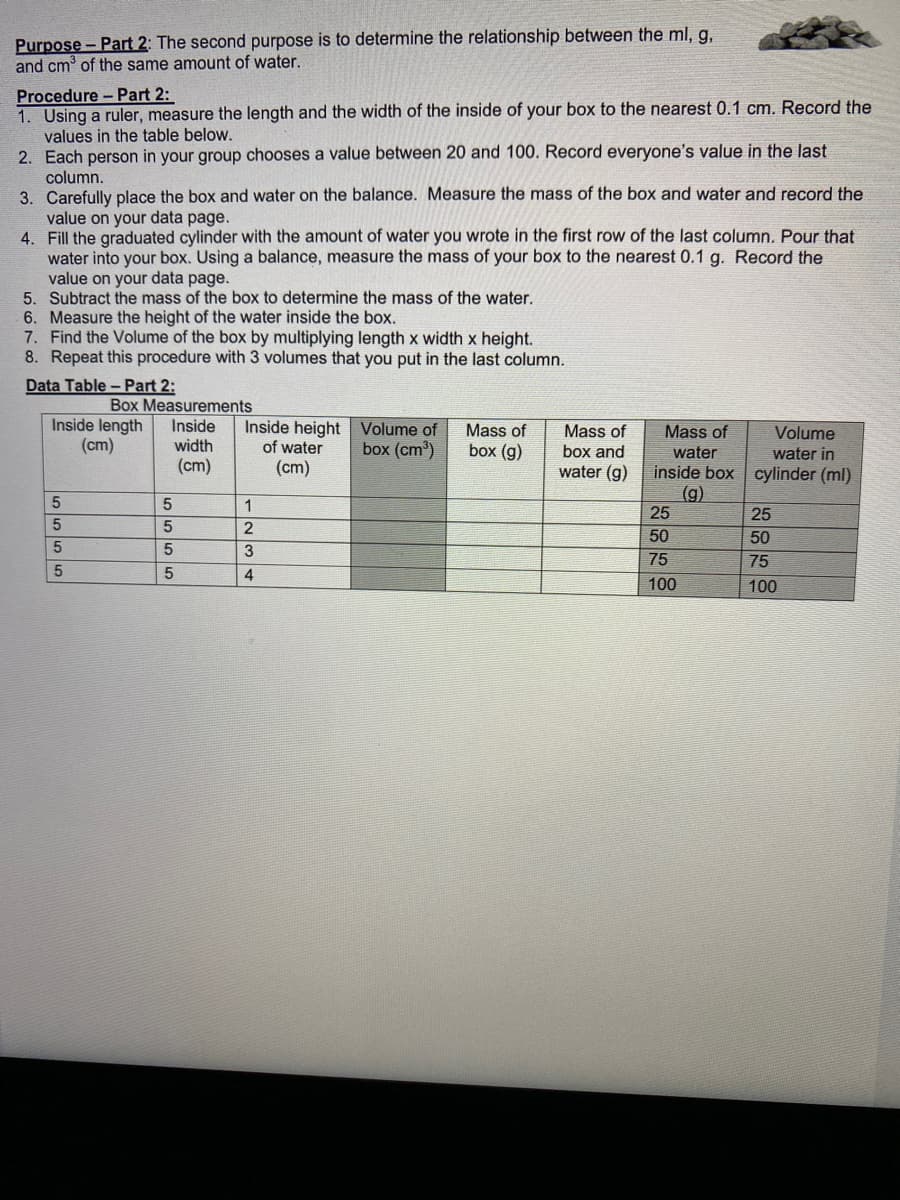
Transcribed Image Text:Purpose - Part 2: The second purpose is to determine the relationship between the ml, g,
and cm³ of the same amount of water.
Procedure - Part 2:
1. Using a ruler, measure the length and the width of the inside of your box to the nearest 0.1 cm. Record the
values in the table below.
2. Each person in your group chooses a value between 20 and 100. Record everyone's value in the last
column.
3. Carefully place the box and water on the balance. Measure the mass of the box and water and record the
value on your data page.
4.
Fill the graduated cylinder with the amount of water you wrote in the first row of the last column. Pour that
water into your box. Using a balance, measure the mass of your box to the nearest 0.1 g. Record the
value on your data page.
5. Subtract the mass of the box to determine the mass of the water.
6. Measure the height of the water inside the box.
7. Find the Volume of the box by multiplying length x width x height.
8. Repeat this procedure with 3 volumes that you put in the last column.
Data Table - Part 2:
Box Measurements
Inside length Inside
(cm)
width
(cm)
5555
5
5
5
5
Inside height
of water
(cm)
1
2
3
4
Volume of
box (cm³)
Mass of
box (g)
Mass of
box and
water (g)
Volume
water in
inside box cylinder (ml)
(g)
Mass of
water
25
50
75
100
25
50
75
100
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 5 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning