Ammonia, NH3, may react with oxygen to form nitrogen gas and water. 4NH3(aq)+302(g)→2N2(g)+6H2O(1) If 3.45 g NH3 reacts with 5.18g 02 and produces 0.950L N2 at 295 K and 1.00 bar, how many moles of N2N2 can be produced by each reactant? It may be useful to consult the periodic table. NH3: mol N2 02: mol N2 Based on the theoretical yields, which reactant is limiting? NH3(aq) 02(g) What is the percent yield of the reaction? percent yield:
Ammonia, NH3, may react with oxygen to form nitrogen gas and water. 4NH3(aq)+302(g)→2N2(g)+6H2O(1) If 3.45 g NH3 reacts with 5.18g 02 and produces 0.950L N2 at 295 K and 1.00 bar, how many moles of N2N2 can be produced by each reactant? It may be useful to consult the periodic table. NH3: mol N2 02: mol N2 Based on the theoretical yields, which reactant is limiting? NH3(aq) 02(g) What is the percent yield of the reaction? percent yield:
Physical Chemistry
2nd Edition
ISBN:9781133958437
Author:Ball, David W. (david Warren), BAER, Tomas
Publisher:Ball, David W. (david Warren), BAER, Tomas
Chapter1: Gases And The Zeroth Law Of Thermodynamics
Section: Chapter Questions
Problem 1.26E: In the anaerobic oxidation of glucose by yeast, CO2 is produced: If 1.56 L of CO2 were produced at...
Related questions
Question
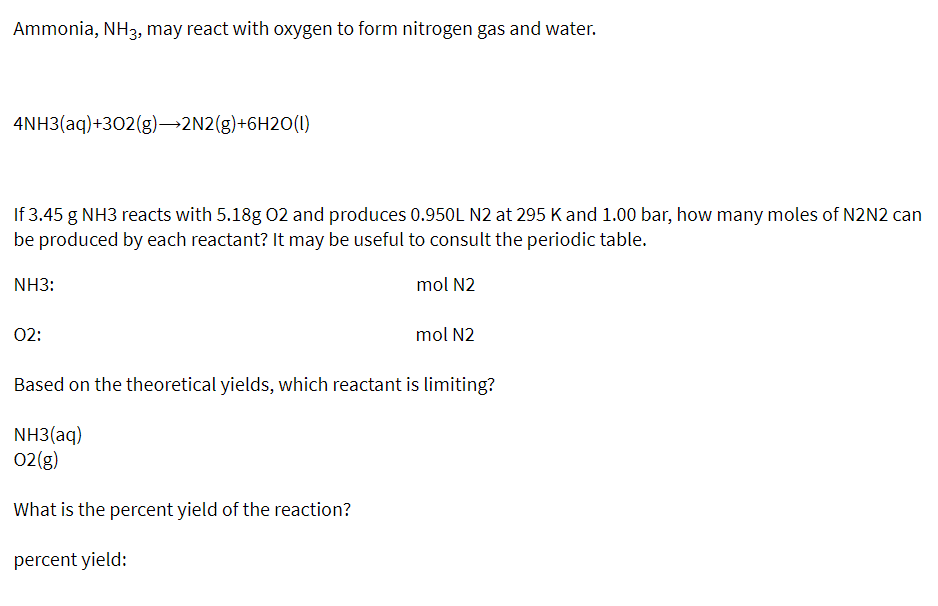
Transcribed Image Text:Ammonia, NH3, may react with oxygen to form nitrogen gas and water.
4NH3(aq)+302(g)→2N2(g)+6H2O(1)
If 3.45 g NH3 reacts with 5.18g 02 and produces 0.950L N2 at 295 K and 1.00 bar, how many moles of N2N2 can
be produced by each reactant? It may be useful to consult the periodic table.
mol N2
NH3:
02:
mol N2
Based on the theoretical yields, which reactant is limiting?
NH3(aq)
O2(g)
What is the percent yield of the reaction?
percent yield:
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 8 steps

Follow-up Questions
Read through expert solutions to related follow-up questions below.
Follow-up Question
hi, sorry the typing is a little cramed together can you seperate the answers
NH3 molN2
O2 molN2
Solution
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

General, Organic, and Biological Chemistry
Chemistry
ISBN:
9781285853918
Author:
H. Stephen Stoker
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning