The structure of the molecule below shows its structure where all of the ionizable/protonatable groups are labeled with their corresponding pKas and are drawn as being in their fully_protonated form. С: рКа- 3.9 OH А: pka - 9.0 OH 'N 'N D: pKa - 3.5 NH H2N NH B: pКа- 12.5 At physiological pH, which of the groups will be deprotonated? O A, B, C, and D O A and B O A and C O Cand D O B and D O A, C, and D What is the total charge of the structure at pH 2? What is the total charge of the structure at pH 10?
The structure of the molecule below shows its structure where all of the ionizable/protonatable groups are labeled with their corresponding pKas and are drawn as being in their fully_protonated form. С: рКа- 3.9 OH А: pka - 9.0 OH 'N 'N D: pKa - 3.5 NH H2N NH B: pКа- 12.5 At physiological pH, which of the groups will be deprotonated? O A, B, C, and D O A and B O A and C O Cand D O B and D O A, C, and D What is the total charge of the structure at pH 2? What is the total charge of the structure at pH 10?
Chapter30: Resolution Of (6)-a-phenylethylamine And Determination Of Optical Purity
Section: Chapter Questions
Problem 1Q
Related questions
Question
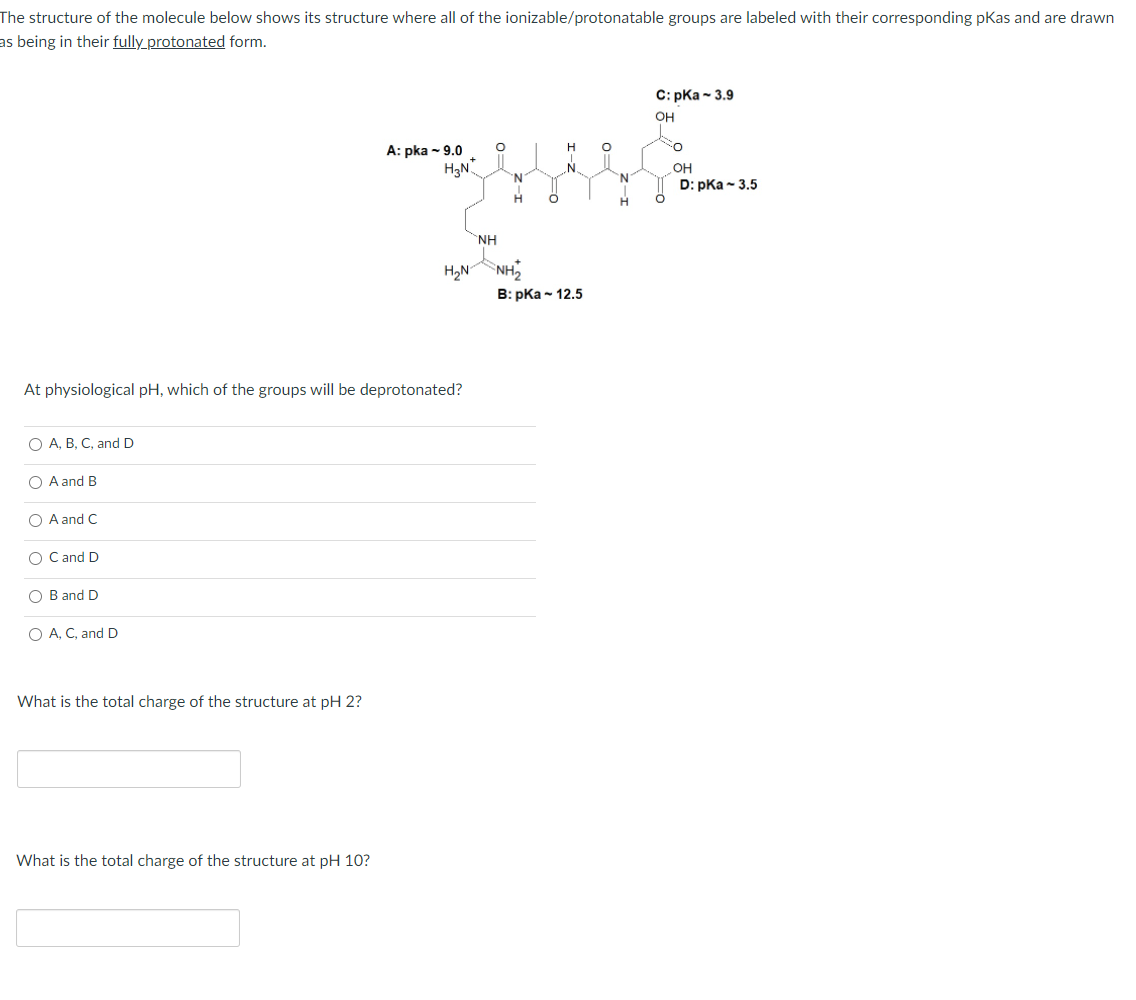
Transcribed Image Text:The structure of the molecule below shows its structure where all of the ionizable/protonatable groups are labeled with their corresponding pKas and are drawn
as being in their fully protonated form.
С: рКa - 3.9
OH
H
А: pka ~ 9.0
H3N
N.
OH
'N.
N.
D: pKa - 3.5
NH
H2NNH,
B: pКa - 12.5
At physiological pH, which of the groups will be deprotonated?
O A, B, C, and D
O A and B
O A and C
O Cand D
O B and D
O A, C, and D
What is the total charge of the structure at pH 2?
What is the total charge of the structure at pH 10?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 4 steps

Recommended textbooks for you

EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:
9781305446021
Author:
Lampman
Publisher:
CENGAGE LEARNING - CONSIGNMENT

EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:
9781305446021
Author:
Lampman
Publisher:
CENGAGE LEARNING - CONSIGNMENT