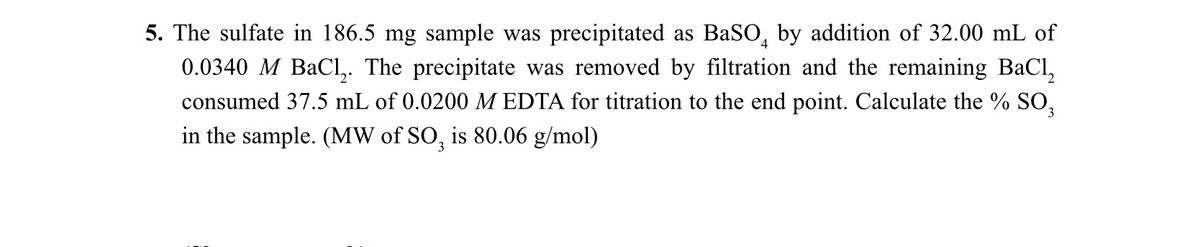
The sulfate in 186.5 mg sample was precipitated as BaSO, by addition of 32.00 mL of 0.0340 M BaCl,. The precipitate was removed by filtration and the remaining BaCl, consumed 37.5 mL of 0.0200 M EDTA for titration to the end point. Calculate the % SO, in the sample. (MW of SO, is 80.06 g/mol)
Q: Determine the molar concentration of an EDTA solution of which 26.44mL, were required to titrate a…
A: In the estimation of calcium ions by EDTA , Ca2+ ion solution is pepared by dissolving CaCO3 in HCl.…
Q: Find the approximate percentage of chlorine, bromine, and iodine in the original sample?
A: Weight of the soluble salts containing chloride, bromide and iodide = 1.2 g The precipitate after…
Q: How would I find the formation constant using the given information?
A: The equilibrium equation for the formation of AC5 complex and the formation constant expression is…
Q: A solution is made by mixing 500.0 mL of 0.04540 M Na, HASO, with 500.0 mL of 0.02245 M NaOH.…
A: Given , molarity of Na2HAsO4= 0.04540 M volume of Na2HAsO4= 500 .0 mL volume of NaOH = 500.0 mL…
Q: The arsenic in a 1.203-g sample was converted to H,AsO4 by suitable treatment. The acid was then…
A: Given data: Weight of pesticide = 1. 203 g Pesticide →H3AsO4→AgNO3 Ag3AsO4 Ag++SCN- →AgSCN(s)…
Q: A 0.64 g sample containing KCI (mw = 74.6 ) is dissolved in 50mL of water and titrated to the…
A: At end point milliequivalents of KCl and milliequivalents of AgNO3 are equal.
Q: The phosphate in a 3.000-g sample of industrial detergent was precipitated by the addition of 1.000…
A: Given Mass of sample - 3 g Mass of AgNO3 = 1g Volume of KSCN = 18.23 ml Molarity of KSCN = 0.1337 M
Q: The Sn in a 0.4352 g mineral specimen was reduced to the +2 state with Pb and titrated with 29.77 mL…
A: Given data: Mass of sample = 0.4352 g Volume of K2Cr2O7 = 29.77 mL Molarity of K2Cr2O7 = 0.01735 M
Q: he sulfur content of insoluble sulfides that do not readilydissolve in acid can be measured by…
A: Given that: mass of sulfur = 5.89 mg moles of Br2 = 1.5 mmol Volume of BaCl2 = 5.000 mL Molarity…
Q: The As in a 9.13-g sample of pesticide was converted to AsO4 3- and precipitated as Ag3AsO4 with…
A: The ratio of mass of particular compound to the total mass of sample multiply by 100 is known as…
Q: Show complete solutions for cach poblem. A seientiet tasked to extract Fe from was an suspension…
A: A) The balanced reactions are as follows: I) Fe2O3(s)+3SO3(g)→Fe2(SO4)3(aq)II)…
Q: The bismuth in 0.7405 g of an alloy was precipitated as BIOCI and separated from the solution by…
A: Solution Given that Alloy weight = 0.7405g AgNO3 required = 12.92…
Q: An unknown sample weighing 0.2583g is titration to the end point with 47.28mL of a 0.10M AgNO3…
A: Given: Mass of sample = 0.2583 g. Volume of AgNO3 solution used = 47.28 mL = 0.04728 L…
Q: To an aqueous solution containing 1.6000 g sample consisting of a mixture of CaBr2•H2O and inert…
A: There are two precipitates in this analysis: AgNO3 and Ag+ form a precipitate of AgNO3 and KSCN…
Q: Calculate the molar solubility of Pbl2(s) in a 0.100 mol/L solution of Nal(aa) at SATP. Ksp = 8. 5 ×…
A: The molar solubility is the product of the concentration of the soluble ions in a solution.…
Q: salt sample was analyzed for its purity. A 0.5000g sample was dissolved in water and diluted to…
A: Given: mass of sample=0.500 gvolume=250 mLmolarity of AgNO3=0.08735 Mmolarity of KSCN=0.09473…
Q: low, ) Nitrite (N07) can be determined by oxidation with excess Ce**, followed by back titration of…
A: Answer
Q: A two-liter sample of mineral water was evaporated to a small volume, following which the potassium…
A:
Q: A salt sample was analyzed for its purity. A 0.5000 g sample was dissolved in water and diluted to…
A: The number of moles of a substance is defined as the mass of the given substance upon its molar…
Q: 4. Silver arsenate, Ag,AsO4, is very insoluble. What weight of Ag,AsO4 precipitate forms when 27.00…
A:
Q: If you want to dissolve 0.225 grams of AgBr, what volume of 0.0138M Na2S2O3 in mililiters, should be…
A: Interpretation - To determine the volume of 0.0138M Na2S2O3 in milliliters when we dissolve 0.225…
Q: ity of saturated solution of of BaSO4 is 3.48 x 10-4 S/m and the conductivity of pure water is 0.50…
A: The ions present in a solution contribute to the total conductivity of a solution. The Ksp for BaSO4…
Q: The sulfur from 4.00g steel is evolved as dihydrogen sulfide gas and titrated with 1.60 mL of…
A:
Q: Cerium(IV) is a strong oxidizing agent commonly used in redox titrations. Which of the following…
A: FACTS about Cerium (IV): A standard solution of Ce4+ is prepared by Cerium (IV) sulfate in 0.5 M…
Q: A. 2.00 ml sample of hydrogen peroxide solution required 8.5ml of a permanganate solution in…
A:
Q: 27) If the 145 mL of a 0.0078 mol/L solution of CuNO3, was mixed with 195 mL of a 1.48 · 10-1mol/L…
A:
Q: The phosphate in a 3.000 g sample of industrial detergent was precipitated by the addition of 1.000…
A: Mass of phosphate sample = 3.0 gram Mass of AgNO3 added = 1.0 gram Required volume of Filterate =…
Q: The arsenic in a 1.208-g sample of a pesticide was converted to H3 AsO4 by suitable treatment. The…
A:
Q: A 0.4000 gram sample of a nickel ote is treated to dissolve the metal. The solution is made basic…
A: The balanced chemical reactions are given below.
Q: A 0.600 g sample containing arsenic (As) was converted to H3AS0, by suitable treatment. 40.00 mL of…
A:
Q: A sample weighing 3.458 g is dissolved and diluted to exactly 250.0 mL in a volumetric flask. A 50.0…
A: Given, A sample weighing 3.458 g is dissolved and diluted to exactly 250.0 mL in a volumetric flask.…
Q: An aqueous ethylene glycol (HOCH, CH, OH, FW = 62.07 g/mol) solution with a mass of 220.9 mg is…
A: Answer: mass percent= 8.15 %
Q: Compare the solubility of silver sulfide in each of the following aqueous solutions
A: “Since you have posted a question with multiple sub-parts, we will solve first three subparts for…
Q: The Sn in a 0.4352 g mineral specimen was reduced to the +2 state and titrated with 29.77 mL of…
A: Given: Mass of specimen = 0.4352 gm Volume of K2Cr2O7 = 29.77 ml Molarity of K2Cr2O7 = 0.01735 M…
Q: Which of the following statement regarding EDTA titration is incorrect * cou bonua inw woled nol…
A: EDTA is a ligand which id hexadentate in nature i.e. has six coordinating sites. It is a very good…
Q: A 0.2420 g sample cntg. Calcium is dissolved and the metal precipitated as CaC,O4 The ppt. is…
A: Solution -
Q: A solution contains 0.0330 M Pb2+ and 0.0210 M Ag+. Can 99% of Pb2+ be precipitated by chromate…
A: Given: Concentration of Pb2+ = 0.0330 M Concentration of Ag+ = 0.0210 M And 99% of Pb2+ needs to be…
Q: The Sn in a 0.4352 g mineral specimen was reduced to the +2 state and titrated with 29.77 mL of…
A: Redox titration is the titration used to determine the analyte concentration by carrying out a redox…
Q: A 0.5g sample of CaCO3 is dissolved in an acidic solution. The calcium is precipitated as…
A: Given Mass of sample = 0.5 g Mass of dry precipitate = 0.72 g
Q: Formula of a compound 261.0 mg of FeCl,-xH,O crystals were dissolved in a diluted hydrochloric acid…
A: FeCl2.xH2O or Fe2+(aq) is oxidized by KMnO4(aq) to FeCl3 or Fe3+(aq). KMnO4(aq) or MnO4-(aq) itself…
Q: Calculate the volume of 0.0723 M EDTA needed to titrate 14.93 mL of 0.0763 M Mg(NO,),.
A:
Q: (a) If the molar solubility of Cu3(PO4)2 at 25 oC is 1.67e-08 mol/L, what is the Ksp at this…
A:
Q: The concentration of CO in the air can be determined by passing a known volume of air through a tube…
A: Parts per million is a commonly used unit of concentration for small values and parts per million…
Q: Which of the following statements is true regarding permanganimetry? a) Permanganate solution can…
A: Permanganate ion, MnO4-is an extremely strong oxidizing agent. It has the ability to oxidize water…
Q: A 20 mL volume of 0.015 M KIO3 containing an excess of KI, is added to a 0.312 g sample of a Real…
A: For given titration reaction the indicator starch is used to identify the end point of the reaction.…
Q: A 20 mL volume of 0.015 M KIO3 containing an excess of KI, is added to a 0.312 g sample of a Real…
A: Given data : A titration between 20 ml volume of 0.015 M KIO3 contains excess iodide with 0.312 g of…
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

- The phosphate in a 3.000-g sample of industrial detergent was precipitated by the addition of 1.000 g of AgNO3. The solution was filtered and filtrate, upon addition of 1.00 mL of 0.01 M fecl3, required 18.23 mL of 0.1377 M KSCN for titration to the end point. (a) the weight percent of phosphate in the detergentA mixture of NaBr, Nal and NaNO3 weighs 0.6500 g. With AgNO3, a precipitate of the two halides is obtained and is found to weigh 0.9390 g. When heated in a current of C2, the precipitate is converted entirely to AgCi weighing 0.6566 g. What is the %NaNO3 in the original sample?The level of dissolved oxygen in a water sample can be determined by the Winkler method. In a typical analysis, a 100.0-mL sample is made basic, and treated with a solution of MnSO4, resulting in the formation of MnO2. An excess of KI is added, and the solution is acidified, resulting in the formation of Mn2+ and I2. The liberated I2 is titrated with a solution of 0.00870 M Na2S2O3, requiring 8.90 mL to reach the starch indicator end point. Calculate the concentration of dissolved oxygen as parts per million of O2.
- Using basic conditions, MnO4- can be used as titrant for the analysis of Mn2+, with both the analyte and the titrant ending up as MnO2. In the analysis of a mineral sample for manganese, a 0.5165-g sample is dissolved, and the manganese is reduced to Mn2+. The solution is made basic and titrated with 0.03358 M KMnO4, requiring 34.88 mL to reach the endpoint. Calculate the %w/w Mn in the mineral sample. Answer: % Mn =The amount of sulfate in a solid sample was determined by first dissolving 562.2 mg of sample in water, and then precipitating the sulfate by the addition of 25.00 mL of 0.022 96 M BaCl2. The precipitate was filtered from the solution and the remaining Ba2+ was titrated with 16.52 mL of 0.014 57 M EDTA. What was the mass percent of sulfate in the solid?The phosphate in a 3.000 g sample of industrial detergent was precipitated by the addition of 1.000 g of AgNO3. The solution was filtered and the filtrate required 18.23 mL of 0.1377 M KSCN for titration to the FeSCN2+ end point. Calculate the percentage of phosphate in the detergent.
- (a) If the molar solubility of Cu3(PO4)2 at 25 oC is 1.67e-08 mol/L, what is the Ksp at this temperature?Ksp = _______(b) It is found that 1.75e-06 g of Cu3(AsO4)2 dissolves per 100 mL of aqueous solution at 25 oC. Calculate the solubility-product constant for Cu3(AsO4)2.Ksp = _______(c) The Ksp of ScF3 at 25 oC is 5.81e-24. What is the molar solubility of ScF3?solubility = ______ mol/LThe concentration of CO in the air can be determined by passing a known volume of air through a tube containing I2O5, resulting in the formation of CO2 and I2. I2 is removed from the tube by distillation and collected in a solution containing excess KI, producing I3-. I3- is titrated with a standard solution of Na2S2O3. A 4.79 L air sample was sampled as described here, requiring 7.17 ml of 0.00329 M Na2S2O3 to reach the endpoint in a typical analysis. If the density of the air is 1.23×10^-3 g/ml, what is the amount of CO in the air in ppm? (CO: 28 g/ml)If you want to dissolve 0.225 grams of AgBr, what volume of 0.0138M Na2S2O3 in mililiters, should be used
- A 20 mL volume of 0.015 M KIO3 containing an excess of KI, is added to a 0.312 g sample of a Real Lemon solution containing vitamin C. The Yellow-brown solution, caused by excess I2 is titrated to a colorless starch endpoint with 11.3 mL pf 0.106M Na2S2O3. - What is the equation for I2 * starch deep blue color disappearance?The arsenic in a 1.22-g sample of a pesticide was converted toAsO43- by suitable chemical treatment. It was then titratedusing Ag+ to form Ag3AsO4 as a precipitate. (a) What is theoxidation state of As in AsO43-? (b) Name Ag3AsO4 by analogyto the corresponding compound containing phosphorusin place of arsenic. (c) If it took 25.0 mL of 0.102 M Ag+to reach the equivalence point in this titration, what is themass percentage of arsenic in the pesticide?A 3.25 g sample of an iron-containing mineral was dissolved in an acid medium and calibrated to 500 mL. A 25.00 mL aliquot was titrated with 0.0025 M KMnO4 spending a volume of 9.32 mL. Subsequently, a 25.00 mL aliquot was passed through a Walden reducer to later titrate it with the same permanganate solution, using a volume of 14.15 mL for the titration. Determine the percentage of Fe(III) in the sample and report it as % Fe2O3

