The vapor pressure of a single component, 2-phase system (vapor is assumed to be ideal gas) was measured using a sealed-end manometer at different temperatures. The data collected are as follows: T (K) 251.2 321.5 286 345 Manometric 36 304 815 1021 pressure (mmHg) With these data. determine the following: a. Enthalpy change of vaporization in kJ/mol | Normal boiling point using data at T = 321.5 K as reference · Is the chemical species of interest vapor, liquid, or both at room b. %3D C. temperature? Explain why.
The vapor pressure of a single component, 2-phase system (vapor is assumed to be ideal gas) was measured using a sealed-end manometer at different temperatures. The data collected are as follows: T (K) 251.2 321.5 286 345 Manometric 36 304 815 1021 pressure (mmHg) With these data. determine the following: a. Enthalpy change of vaporization in kJ/mol | Normal boiling point using data at T = 321.5 K as reference · Is the chemical species of interest vapor, liquid, or both at room b. %3D C. temperature? Explain why.
Physical Chemistry
2nd Edition
ISBN:9781133958437
Author:Ball, David W. (david Warren), BAER, Tomas
Publisher:Ball, David W. (david Warren), BAER, Tomas
Chapter6: Equilibria In Single-component Systems
Section: Chapter Questions
Problem 6.50E
Related questions
Question
*4decimals final answers
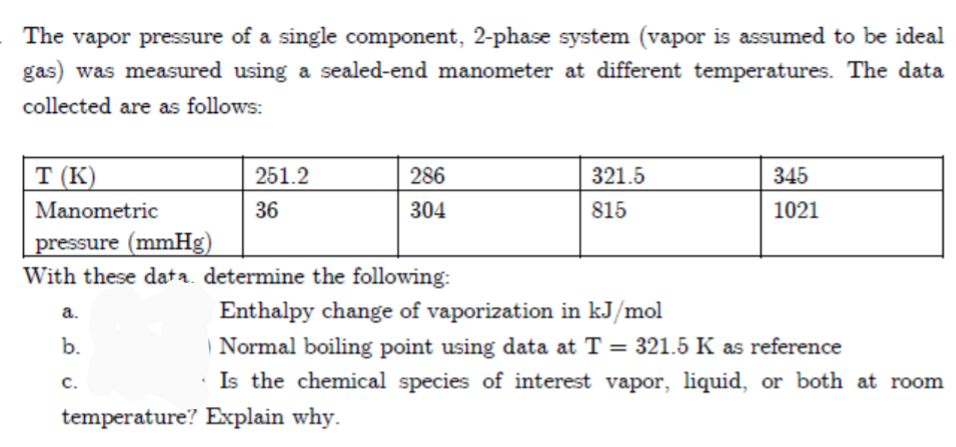
Transcribed Image Text:The vapor pressure of a single component, 2-phase system (vapor is assumed to be ideal
gas) was measured using a sealed-end manometer at different temperatures. The data
collected are as follows:
T (K)
251.2
286
321.5
345
Manometric
36
304
815
1021
pressure (mmHg)
With these data. determine the following:
Enthalpy change of vaporization in kJ/mol
a.
b.
| Normal boiling point using data at T = 321.5 K as reference
с.
· Is the chemical species of interest vapor, liquid, or both at room
temperature? Explain why.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 4 steps with 1 images

Recommended textbooks for you

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,