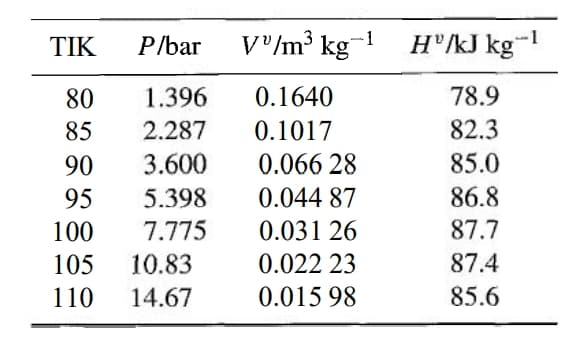
Liquid nitrogen is stored in 0.5-m3 metal tanks that are thoroughly insulated. Consider the process of filling an evacuated tank, initially at 295 K. It is attached to a line containing liquid nitrogen at its normal boiling point of 77.3 K and at a pressure of several bars. At this condition, its enthalpy is -120.8 kJ kg-1. When a valve in the line is opened, the nitrogen flowing into the tank at first evaporates in the process of cooling the tank. If the tank has a mass of 30 kg and the metal has a specific heat capacity of 0.43 kJ kg-1 K-1, what mass of nitrogen must flow into the tank just to cool it to a temperature such that liquid nitrogen begins to accumulate in the tank? Assume that the nitrogen and the tank are always at the same temperature. The properties of saturated nitrogen vapor at several temperatures are given as follows:
Liquid nitrogen is stored in 0.5-m3 metal tanks that are thoroughly insulated. Consider the process of filling an evacuated tank, initially at 295 K. It is attached to a line containing liquid nitrogen at its normal boiling point of 77.3 K and at a pressure of several bars. At this condition, its enthalpy is -120.8 kJ kg-1. When a valve in the line is opened, the nitrogen flowing into the tank at first evaporates in the process of cooling the tank. If the tank has a mass of 30 kg and the metal has a specific heat capacity of
0.43 kJ kg-1 K-1, what mass of nitrogen must flow into the tank just to cool it to a temperature such that liquid nitrogen begins to accumulate in the tank? Assume that the nitrogen and the tank are always at the same temperature. The properties of saturated nitrogen vapor at several temperatures are given as follows:
Trending now
This is a popular solution!
Step by step
Solved in 3 steps









