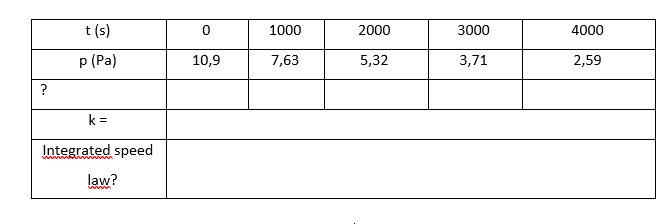
The variation in the partial pressure of azomethane (Azo) with time was followed at 600 K and the data obtained are reproduced in the table below. a) Confirm that the decomposition of CH3N2CH3(g) C2H6(g) + N2(g) is first order with respect to azomethane and find the rate constant (reaction rate constant) at the temperature of the experiment. Interpret your answer. b) Fill in the table with the values and information you consider necessary to resolve the questions asked. c) What is the integrated form of the velocity law? (1.0 Pa ≈ 1.0 x 10-5 atm; 1.0 atm = 101.325 kPa); p = partial pressure).
The variation in the partial pressure of azomethane (Azo) with time was followed at 600 K and the data obtained are reproduced in the table below. a) Confirm that the decomposition of CH3N2CH3(g) C2H6(g) + N2(g) is first order with respect to azomethane and find the rate constant (reaction rate constant) at the temperature of the experiment. Interpret your answer. b) Fill in the table with the values and information you consider necessary to resolve the questions asked. c) What is the integrated form of the velocity law? (1.0 Pa ≈ 1.0 x 10-5 atm; 1.0 atm = 101.325 kPa); p = partial pressure).
Chapter6: Random Errors In Chemical Analysis
Section: Chapter Questions
Problem 6.22QAP
Related questions
Question
The variation in the partial pressure of azomethane (Azo) with time was followed at 600 K and the data obtained are reproduced in the table below.
a) Confirm that the decomposition of CH3N2CH3(g) C2H6(g) + N2(g) is first order with respect to azomethane and find the rate constant (reaction rate constant) at the temperature of the experiment. Interpret your answer.
b) Fill in the table with the values and information you consider necessary to resolve the questions asked.
c) What is the integrated form of the velocity law? (1.0 Pa ≈ 1.0 x 10-5 atm; 1.0 atm = 101.325 kPa); p = partial pressure).
Transcribed Image Text:t (s)
1000
2000
3000
4000
p (Pa)
10,9
7,63
5,32
3,71
2,59
k =
Integrated speed
law?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 6 steps with 1 images

Recommended textbooks for you

