The hydrogenation of acetylene (C2H2) on Pd metal sites (*) is proposed to occur via elementary steps consisting of: 1) QE dissociative adsorption of H2: 2) QE molecular adsorption of C2H2; 3) & 4) QE sequential addition of H" to form C:Ha"; 5) Irreversible desorption of ethylene. Write the mechanism of the reaction with the stoichiometric number of each step. Derive the rate expression in terms of constants and measurable quantities when C:H," and " are the MARI. If C;H, is in the MARI expression, why is the rate independent of the concentration of C¿H4 in the gas phase? At some conditions, the experimental rate is zero order in both CH2 and H2. What additional assumption(s) are required for this observation to be consistent with the rate expression derived in part (b)? 1) If, under certain experimental conditions, an effective zero order rate constant is measured, explain the chemical significance of this constant (in terms of rate or equilibrium constants for elementary steps) using the rate expression in part (b).
The hydrogenation of acetylene (C2H2) on Pd metal sites (*) is proposed to occur via elementary steps consisting of: 1) QE dissociative adsorption of H2: 2) QE molecular adsorption of C2H2; 3) & 4) QE sequential addition of H" to form C:Ha"; 5) Irreversible desorption of ethylene. Write the mechanism of the reaction with the stoichiometric number of each step. Derive the rate expression in terms of constants and measurable quantities when C:H," and " are the MARI. If C;H, is in the MARI expression, why is the rate independent of the concentration of C¿H4 in the gas phase? At some conditions, the experimental rate is zero order in both CH2 and H2. What additional assumption(s) are required for this observation to be consistent with the rate expression derived in part (b)? 1) If, under certain experimental conditions, an effective zero order rate constant is measured, explain the chemical significance of this constant (in terms of rate or equilibrium constants for elementary steps) using the rate expression in part (b).
Organic Chemistry
8th Edition
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Chapter8: Haloalkanes, Halogenation, And Radical Reactions
Section: Chapter Questions
Problem 8.34P
Related questions
Question
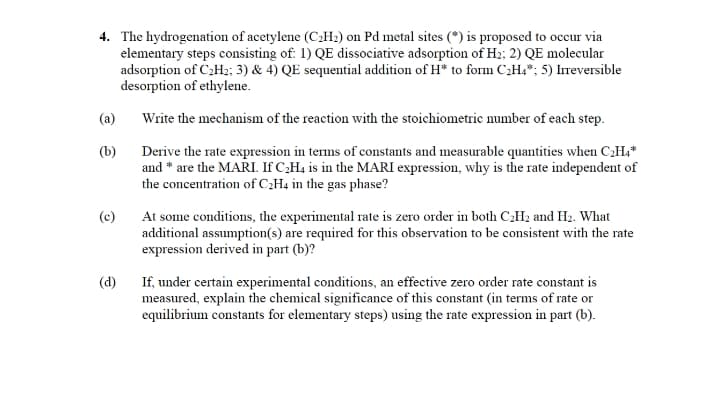
Transcribed Image Text:4. The hydrogenation of acetylene (C2H2) on Pd metal sites (*) is proposed to occur via
elementary steps consisting of: 1) QE dissociative adsorption of H2; 2) QE molecular
adsorption of C2H2; 3) & 4) QE sequential addition of H* to form C2H4*; 5) Ireversible
desorption of ethylene.
(a)
Write the mechanism of the reaction with the stoichiometric number of each step.
(b)
Derive the rate expression in tems of constants and measurable quantities when CH,*
and * are the MARI. If C;H4 is in the MARI expression, why is the rate independent of
the concentration of C¿H4 in the gas phase?
(c)
At some conditions, the experimental rate is zero order in both CH2 and H2. What
additional assumption(s) are required for this observation to be consistent with the rate
expression derived in part (b)?
If, under certain experimental conditions, an effective zero order rate constant is
measured, explain the chemical significance of this constant (in terms of rate or
equilibrium constants for elementary steps) using the rate expression in part (b).
(d)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning


Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning

Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning


Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning