There has been increased evidence that human activities are causing changes in Earth's atmospheric chemistry. Recent research efforts have focused on atmospheric ozone (03) concentrations. The amount of ozone in the atmosphere is influenced by concentrations of gases that contain only nitrogen and oxygen, among others. The following table gives the masses of nitrogen that combine with 1.00 g of oxygen to form three of these compounds. Compound Mass of Nitrogen A 0.875 B 0.438 0.350 a. Determine the ratios of the masses of nitrogen that combine with 1.00 g of oxygen in these compounds. A/C =[O B/C = A/B =
There has been increased evidence that human activities are causing changes in Earth's atmospheric chemistry. Recent research efforts have focused on atmospheric ozone (03) concentrations. The amount of ozone in the atmosphere is influenced by concentrations of gases that contain only nitrogen and oxygen, among others. The following table gives the masses of nitrogen that combine with 1.00 g of oxygen to form three of these compounds. Compound Mass of Nitrogen A 0.875 B 0.438 0.350 a. Determine the ratios of the masses of nitrogen that combine with 1.00 g of oxygen in these compounds. A/C =[O B/C = A/B =
Chemistry: Principles and Reactions
8th Edition
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:William L. Masterton, Cecile N. Hurley
Chapter3: Mass Relations In Chemistry; Stoichiometry
Section: Chapter Questions
Problem 42QAP: Saccharin is the active ingredient in many sweeteners used today. It is made up of carbon, hydrogen,...
Related questions
Question

Transcribed Image Text:There has been increased evidence that human activities are causing changes in Earth's atmospheric chemistry. Recent research efforts have focused on atmospheric ozone (O3) concentrations.
The amount of ozone in the atmosphere is influenced by concentrations of gases that contain only nitrogen and oxygen, among others. The following table gives the masses of nitrogen that combine
with 1.00 g of oxygen to form three of these compounds.
Compound Mass of Nitrogen
A
0.875
B
0.438
C
0.350
a. Determine the ratios of the masses of nitrogen that combine with 1.00 g of oxygen in these compounds.
A/C =
B/C =
A/B =
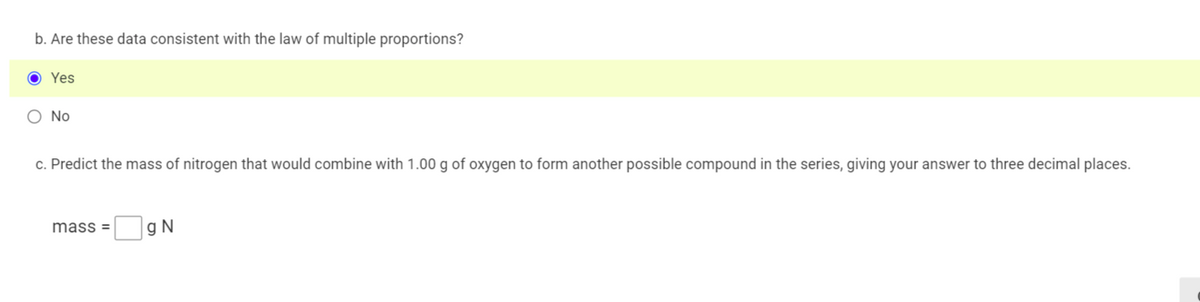
Transcribed Image Text:b. Are these data consistent with the law of multiple proportions?
Yes
O No
c. Predict the mass of nitrogen that would combine with 1.00 g of oxygen to form another possible compound in the series, giving your answer to three decimal places.
mass =
g N
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps

Recommended textbooks for you

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781285199030
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning