Thermochemistry 1 Name Tbp AH fus ΔΗ C solid C liquid C gas vap (°C) (°C) (J/g) (kJ/mol) (J/ go°C) (J/ g•°C) (J/ go°C) H2O Water 0.0 100.0 333.55 40.65 2.06 4.184 1.87 HC2H3O2 Acetic Acid CH3CH2OH Ethanol 16.0 118.1 194.8 23.7 N/A 2.05 1.06 -117 78 109 38.56 0.97 2.3 0.95 C solid (J/ go°C) 0.902 Metal Aluminum 0.385 Соpper Iron 0.444 Silver 0.240 Zinc 0.39 1. Calculate the energy released when 25 g of water cools from a gas 100.0 °C to a liquid at 56.5 °C. F-I Q= m CAT =(259)(1.87 J/9.°C)( 5u5'c- 10.0C) 2. What mass of water in grams can be heated from 25.0 °C to 33.5 °C by the heat energy released when a 50.0 g chunk of aluminum cools from 100.0 °C to 33.5 °C? 3. Calculate the heat required to convert 40.0 g of acetic acid, HC2H;O2, from a solid at 16.0 °C to a liquid at 99.9 °C. CS Scanned with CamScanner
Thermochemistry 1 Name Tbp AH fus ΔΗ C solid C liquid C gas vap (°C) (°C) (J/g) (kJ/mol) (J/ go°C) (J/ g•°C) (J/ go°C) H2O Water 0.0 100.0 333.55 40.65 2.06 4.184 1.87 HC2H3O2 Acetic Acid CH3CH2OH Ethanol 16.0 118.1 194.8 23.7 N/A 2.05 1.06 -117 78 109 38.56 0.97 2.3 0.95 C solid (J/ go°C) 0.902 Metal Aluminum 0.385 Соpper Iron 0.444 Silver 0.240 Zinc 0.39 1. Calculate the energy released when 25 g of water cools from a gas 100.0 °C to a liquid at 56.5 °C. F-I Q= m CAT =(259)(1.87 J/9.°C)( 5u5'c- 10.0C) 2. What mass of water in grams can be heated from 25.0 °C to 33.5 °C by the heat energy released when a 50.0 g chunk of aluminum cools from 100.0 °C to 33.5 °C? 3. Calculate the heat required to convert 40.0 g of acetic acid, HC2H;O2, from a solid at 16.0 °C to a liquid at 99.9 °C. CS Scanned with CamScanner
Chemistry: Principles and Practice
3rd Edition
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Chapter5: Thermochemistry
Section: Chapter Questions
Problem 5.103QE
Related questions
Question
can you help me solve #2?
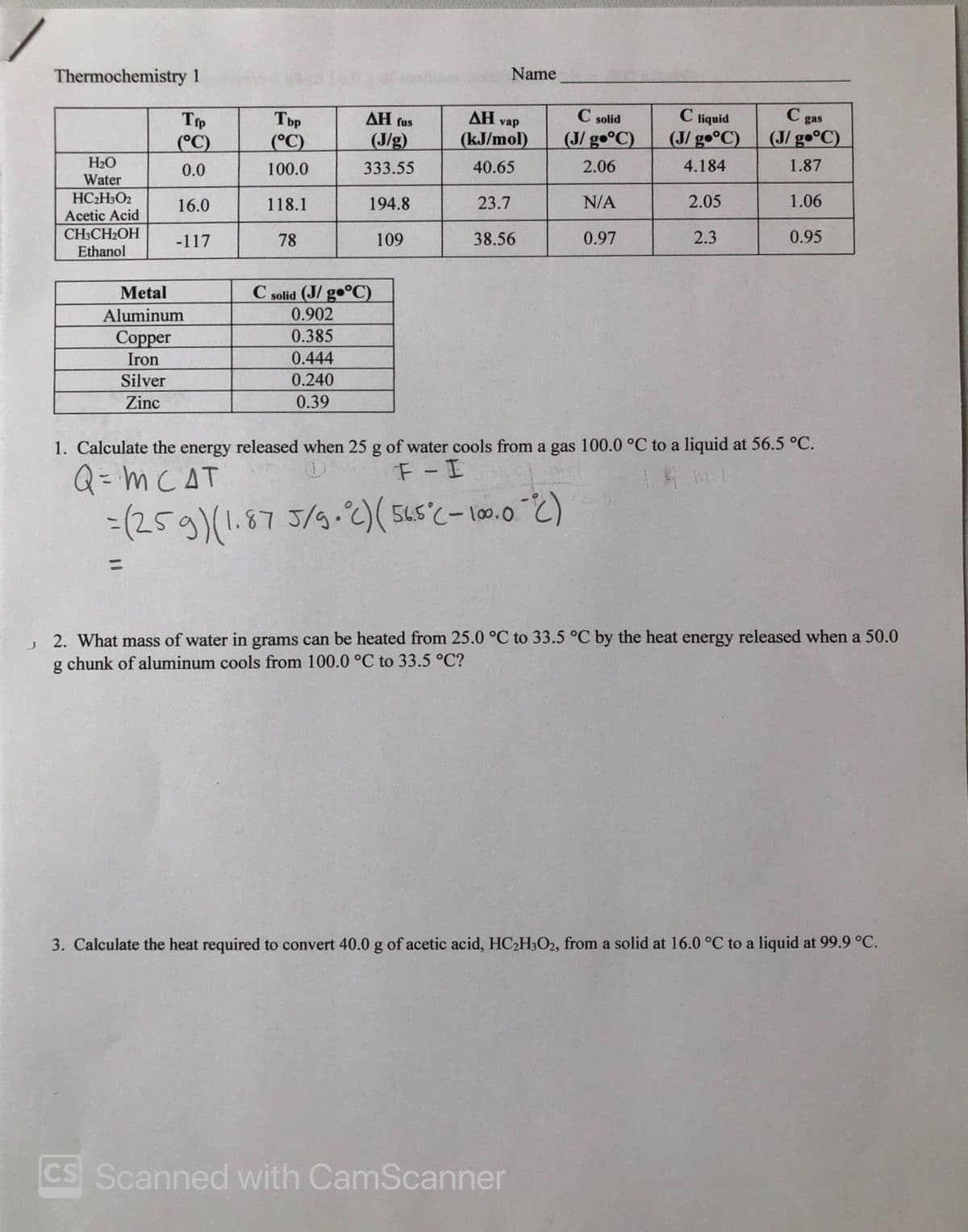
Transcribed Image Text:Thermochemistry 1
Name
AH vap
C solid
C liquid
(J/ gº°C)
C gas
(J/ g•°C)
Tbp
AH fus
Тр
(°C)
(J/ g•°C)
(°C)
(J/g)
(kJ/mol)
H2O
0.0
100.0
333.55
40.65
2.06
4.184
1.87
Water
HC2H3O2
Acetic Acid
16.0
118.1
194.8
23.7
N/A
2.05
1.06
CH3CH2OH
Ethanol
-117
78
109
38.56
0.97
2.3
0.95
C solid (J/ go°C)
0.902
Metal
Aluminum
0.385
Соpper
Iron
0.444
Silver
0.240
Zinc
0.39
1. Calculate the energy released when 25 g of water cools from a gas 100.0 °C to a liquid at 56.5 °C.
F-I
Q= m CAT
-(259)(1.87 5/9-2)( 5Ls'c- 10.0 2)
J 2. What mass of water in grams can be heated from 25.0 °C to 33.5 °C by the heat energy released when a 50.0
g chunk of aluminum cools from 100.0 °C to 33.5 °C?
3. Calculate the heat required to convert 40.0 g of acetic acid, HC2H3O2, from a solid at 16.0 °C to a liquid at 99.9 °C.
CS Scanned with CamScanner
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning