This energy diagram shows the allowed energy levels of an electron in a certain atom or molecule: D energy Use this diagram to complete the table below. Which is the ground state? (pick one) e How many excited states are there? How many lines are in the absorption line spectrum? Which transition causes the absorption line at the shortest wavelength? Which transition causes the absorption line at the longest wavelength?
This energy diagram shows the allowed energy levels of an electron in a certain atom or molecule: D energy Use this diagram to complete the table below. Which is the ground state? (pick one) e How many excited states are there? How many lines are in the absorption line spectrum? Which transition causes the absorption line at the shortest wavelength? Which transition causes the absorption line at the longest wavelength?
Chemistry: Principles and Reactions
8th Edition
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:William L. Masterton, Cecile N. Hurley
Chapter6: Electronic Structure And The Periodic Table
Section: Chapter Questions
Problem 63QAP: A carbon dioxide laser produces radiation of wavelength 10.6 micrometers (1micrometer=106meter)....
Related questions
Question
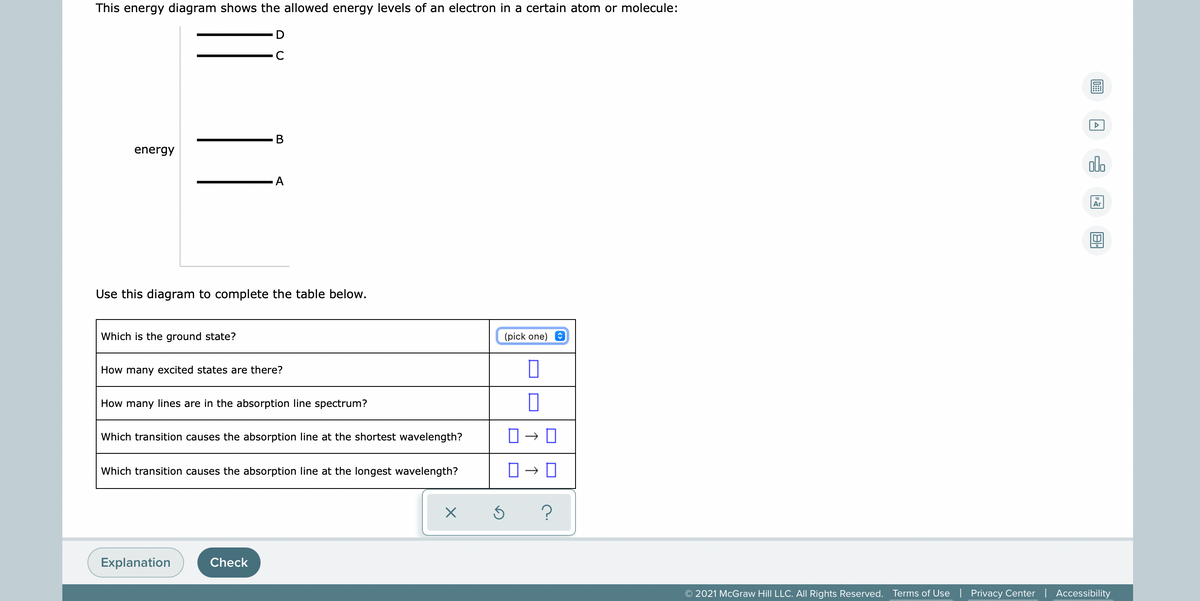
Transcribed Image Text:This energy diagram shows the allowed energy levels of an electron in a certain atom or molecule:
energy
olo
18
Ar
Use this diagram to complete the table below.
Which is the ground state?
(pick one)
How many excited states are there?
How many lines are in the absorption line spectrum?
Which transition causes the absorption line at the shortest wavelength?
Which transition causes the absorption line at the longest wavelength?
Explanation
Check
© 2021 McGraw Hill LLC. AlI Rights Reserved.
Terms of Use | Privacy Center | Accessibility
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Recommended textbooks for you

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,