This question concerns the changes which take place when a 0.1M solution of sodium hydroxide is added to 25cm3 of 0.1M hydrochloric acid. In 3 separate experiments, changes in temperature, pH, and electrical conductivity did take place in the cell, respectively. On the axes below, use broken lines(------) to indicate the results you would expect if 0.1M ethanoic acid were used instead of hydrochloric acid. Temperature arbitrary scale against Volume of alkali in cm3 pH against alkali in cm3 Conductivity against alkali in cm3
This question concerns the changes which take place when a 0.1M solution of sodium hydroxide is added to 25cm3 of 0.1M hydrochloric acid. In 3 separate experiments, changes in temperature, pH, and electrical conductivity did take place in the cell, respectively. On the axes below, use broken lines(------) to indicate the results you would expect if 0.1M ethanoic acid were used instead of hydrochloric acid. Temperature arbitrary scale against Volume of alkali in cm3 pH against alkali in cm3 Conductivity against alkali in cm3
Principles of Instrumental Analysis
7th Edition
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Chapter33: Automated Methods Of Analysis
Section: Chapter Questions
Problem 33.6QAP
Related questions
Question
This question concerns the changes which take place when a 0.1M solution of sodium hydroxide is added to 25cm3 of 0.1M hydrochloric acid.
In 3 separate experiments, changes in temperature, pH, and electrical conductivity did take place in the cell, respectively.
On the axes below, use broken lines(------) to indicate the results you would expect if 0.1M ethanoic acid were used instead of hydrochloric acid.
- Temperature arbitrary scale against Volume of alkali in cm3
- pH against alkali in cm3
- Conductivity against alkali in cm3
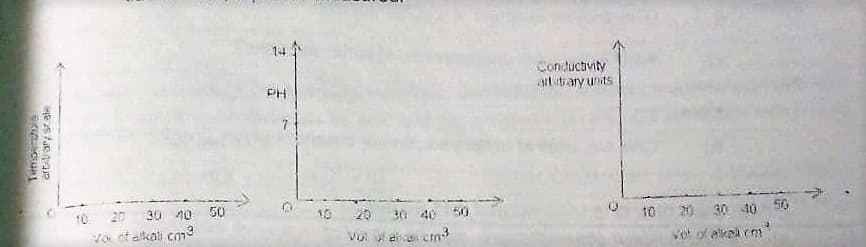
Transcribed Image Text:Conductvity
artitrary unitS
20
30 10 50
10
20
30 40
50
10
20)
30 40 0
vo of akai cm3
cta3
vol of alkei cm
Vo
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 4 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning