This table is all about ions. If you're not told the ionic charge, make it what charge to forms when it becomes an ion. Element Name magnesium ion fluorine ion rhodium (III) ion Element Symbol Zn-² #protons 34 # electrons #neutrons 45
This table is all about ions. If you're not told the ionic charge, make it what charge to forms when it becomes an ion. Element Name magnesium ion fluorine ion rhodium (III) ion Element Symbol Zn-² #protons 34 # electrons #neutrons 45
ChapterU1: Alchemy: Matter, Atomic Structure, And Bonding
Section: Chapter Questions
Problem 3STP
Related questions
Question
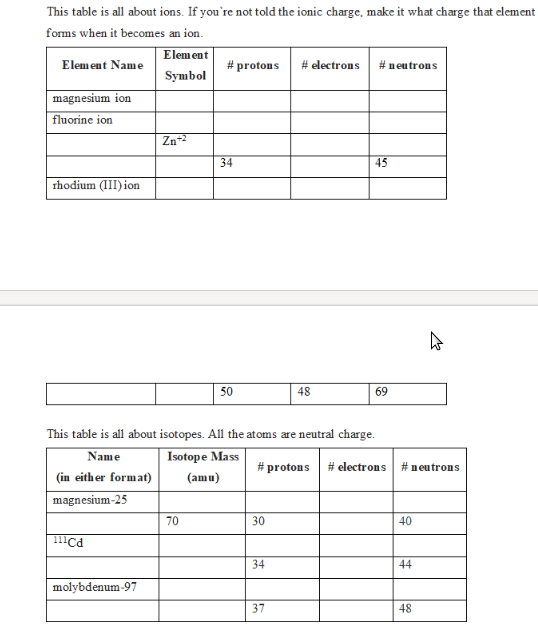
Transcribed Image Text:This table is all about ions. If you're not told the ionic charge, make it what charge that element
forms when it becomes an ion.
Element Name
magnesium ion
fluorine ion
rhodium (III)ion
(in either format)
magnesium-25
111cd
Element
Symbol
molybdenum-97
Zn +²
#protons
70
34
50
This table is all about isotopes. All the atoms are neutral charge.
Name
Isotope Mass
#protons # electrons
(amu)
30
34
# electrons
37
48
#neutrons
45
69
#neutrons
40
44
48
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning



Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning