Tiny samples of aqueous solutions are sketched below, as if under a microscope so powerful that individual molecules could be seen. (The water molecules are not shown.) The two substances in each sample can interconvert. That is, each kind of molecule can turn into the other. The equilibrium constant K for each interconversion equilibrium is shown below the sketch. Decide whether each solution is at equilibrium. K At equilibrium? O no Oyes 32 88 K-11 At equilibrium? O yes Ono
Tiny samples of aqueous solutions are sketched below, as if under a microscope so powerful that individual molecules could be seen. (The water molecules are not shown.) The two substances in each sample can interconvert. That is, each kind of molecule can turn into the other. The equilibrium constant K for each interconversion equilibrium is shown below the sketch. Decide whether each solution is at equilibrium. K At equilibrium? O no Oyes 32 88 K-11 At equilibrium? O yes Ono
Chemistry: Principles and Reactions
8th Edition
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:William L. Masterton, Cecile N. Hurley
Chapter12: Gaseous Chemical Equilibrium
Section: Chapter Questions
Problem 36QAP: At a certain temperature, K=0.29 for the decomposition of two moles of iodine trichloride, ICl3(s),...
Related questions
Question
Give typed explanation not written
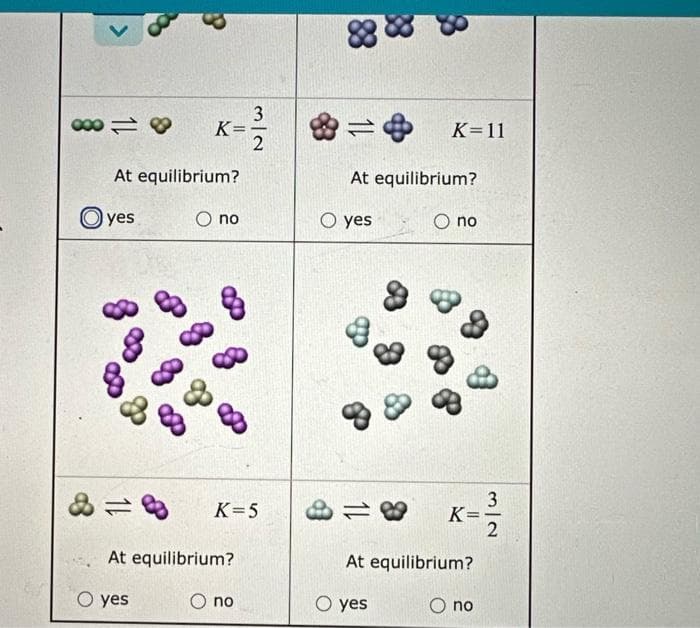
Transcribed Image Text:00018
11
3
At equilibrium?
yes
O yes
K=
O no
8
8
At equilibrium?
O no
3/2
K=5
88
O yes
At equilibrium?
11
38
K=11
O yes
O no
8 K=
At equilibrium?
O no
3/2
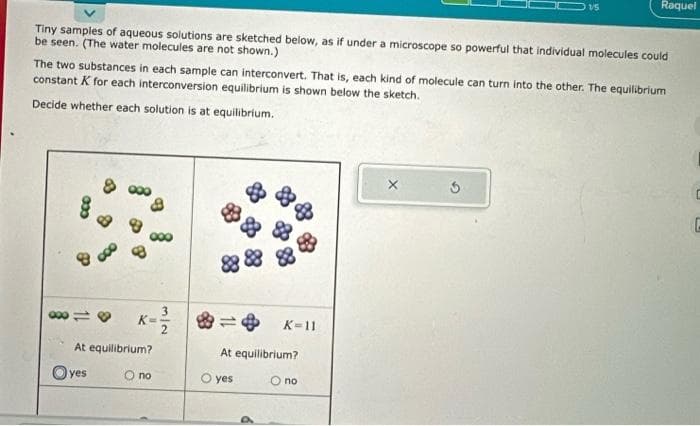
Transcribed Image Text:Tiny samples of aqueous solutions are sketched below, as if under a microscope so powerful that individual molecules could
be seen. (The water molecules are not shown.)
033
The two substances in each sample can interconvert. That is, each kind of molecule can turn into the other. The equilibrium
constant K for each interconversion equilibrium is shown below the sketch.
Decide whether each solution is at equilibrium.
80118 K=
At equilibrium?
O no
yes
015
K-11
At equilibrium?
O no
O yes
Raquel
G
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 5 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning