to answer questions 14-17. :F : H H Si H FC :F : H. H H H A В 14. Which molecule(s) is/are polar? A) A В) В С) С D) B and C E) A and C 15. What is the strongest type of intermolecular force present in B? A) Ion-dipole B) London dispersion C) Hydrogen bonding D) Dipole-dipole E) None of the above
to answer questions 14-17. :F : H H Si H FC :F : H. H H H A В 14. Which molecule(s) is/are polar? A) A В) В С) С D) B and C E) A and C 15. What is the strongest type of intermolecular force present in B? A) Ion-dipole B) London dispersion C) Hydrogen bonding D) Dipole-dipole E) None of the above
Introductory Chemistry: A Foundation
9th Edition
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Donald J. DeCoste
Chapter14: Liquids And Solids
Section: Chapter Questions
Problem 16ALQ: True or false? Methane (CH4) is more likely In form stronger hydrogen bonding than is water because...
Related questions
Question
#15
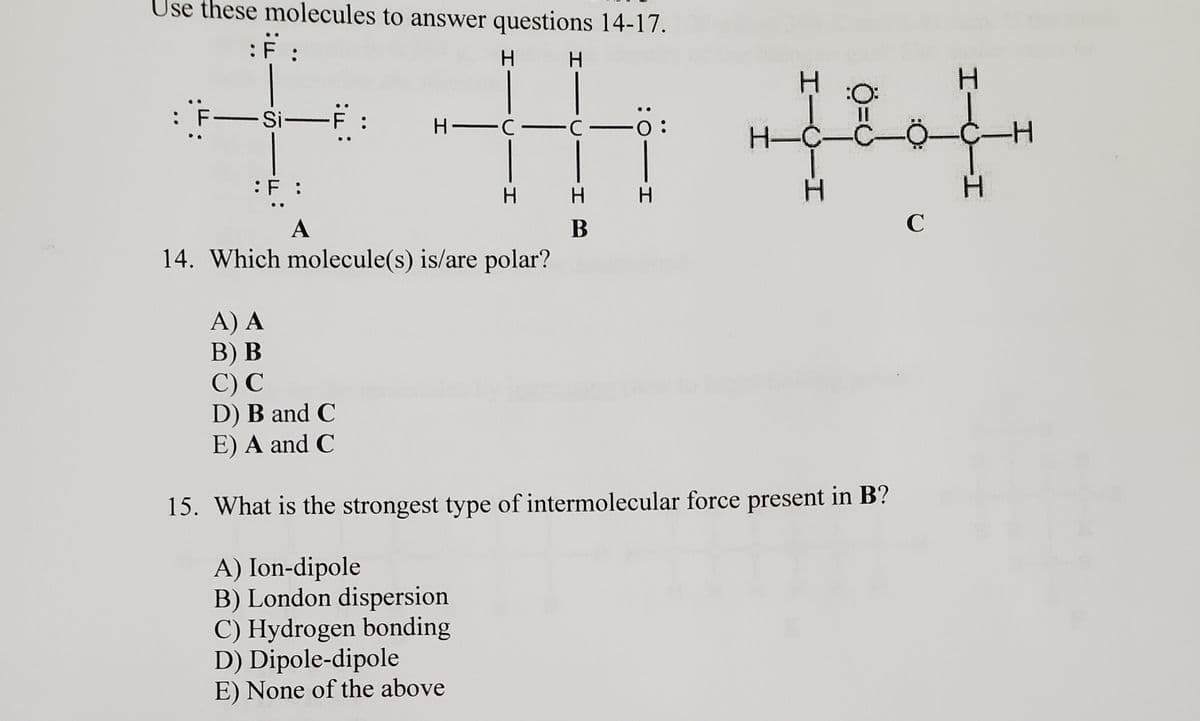
Transcribed Image Text:Use these molecules to answer questions 14-17.
:F :
H.
H.
:O:
Si-F :
:
H -C-C -0:
H-C-C
-
:F :
H.
H.
H.
A
В
14. Which molecule(s) is/are polar?
A) A
В) В
C) С
D) B and C
E) A and C
15. What is the strongest type of intermolecular force present in B?
A) Ion-dipole
B) London dispersion
C) Hydrogen bonding
D) Dipole-dipole
E) None of the above
Expert Solution
Introduction.
- The polarity of a molecule is dependent on the electronegativity difference of atoms and geometry of molecule.
- In SF4 , the S-F bond is polar but the geometry of molecule is tetrahedral and thus the resultant dipole moment is zero, So, SF4 is non polar.
- The O-H bond is alcohols is a polar bond which ,makes the alcohol molecule polar.
- Ester molecules are polar due to electronegativity difference between C and O.
Part 14
Polar molecules among the given molecules are: Ethanol and Methyl ethanoate.
Hence, the correct option is:
D) B and C
Step by step
Solved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781285199023
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781285199023
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

