To determine the amount of a hypothetical compound KHA. H2A 2H2O in a pure sample, Senku asked his friend Chrome to titrate it with 0.100M NaOH. Unfortunately, an accident occurred while he was away and all of the 50.0 mL NaOH solution was mixed into the sample. To remedy the situation, Senku decided to perform back titration with a 2.375 mL of 0.0800M H2SO4 solution. a) How many excess moles of NaOH were added to the solution? b) How many moles of the compound KHA.H2A.2H2O are present in the sample? c) When the reaction between the same sample of KHA.H2A.2H2O and NaOH was carried out in a coffee cup calorimeter, the measured total temperature rise from 25.0°C was 54.0°C. Determine the magnitude of the heat of the neutralization of KHA.H2A.2H2O and NaOH in Joules. Assume that the Cp of the solution is 18.0 cal/mol-K.
To determine the amount of a hypothetical compound KHA. H2A 2H2O in a pure sample, Senku asked his friend Chrome to titrate it with 0.100M NaOH. Unfortunately, an accident occurred while he was away and all of the 50.0 mL NaOH solution was mixed into the sample. To remedy the situation, Senku decided to perform back titration with a 2.375 mL of 0.0800M H2SO4 solution. a) How many excess moles of NaOH were added to the solution? b) How many moles of the compound KHA.H2A.2H2O are present in the sample? c) When the reaction between the same sample of KHA.H2A.2H2O and NaOH was carried out in a coffee cup calorimeter, the measured total temperature rise from 25.0°C was 54.0°C. Determine the magnitude of the heat of the neutralization of KHA.H2A.2H2O and NaOH in Joules. Assume that the Cp of the solution is 18.0 cal/mol-K.
Chemistry
10th Edition
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Chapter15: Acid-base Equilibria
Section: Chapter Questions
Problem 128CP: Consider the titration curve in Exercise 115 for the titration of Na2Cr3 with HCl. a. If a mixture...
Related questions
Question
Solve for b) and c)
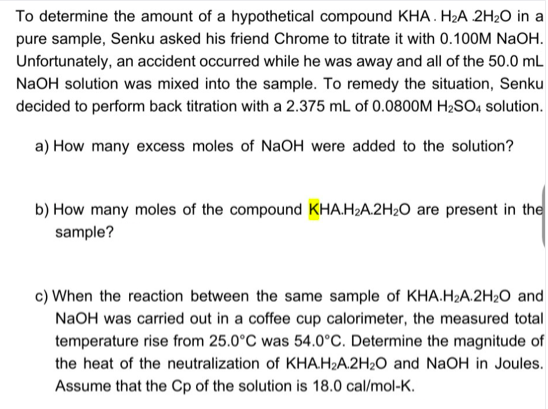
Transcribed Image Text:To determine the amount of a hypothetical compound KHA. H2A 2H20 in a
pure sample, Senku asked his friend Chrome to titrate it with 0.100M NaOH.
Unfortunately, an accident occurred while he was away and all of the 50.0 mL
NaOH solution was mixed into the sample. To remedy the situation, Senku
decided to perform back titration with a 2.375 mL of 0.0800OM H2SO, solution.
a) How many excess moles of NaOH were added to the solution?
b) How many moles of the compound KHA.H2A.2H2O are present in the
sample?
c) When the reaction between the same sample of KHA.H2A.2H2O and
NaOH was carried out in a coffee cup calorimeter, the measured total
temperature rise from 25.0°C was 54.0°C. Determine the magnitude of
the heat of the neutralization of KHA.H2A.2H2O and NaOH in Joules.
Assume that the Cp of the solution is 18.0 cal/mol-K.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning



Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning