A www-awn.aleks.com Significant Figures Counter Chapter 5. The Nervous System-Neurolo.. https://learn-us-east-1-prod-fleet01-xyth... ALEKS Sabina Lamgadhey - Learn O CHEMICAL REACTIONS Solving for a reactant using a chemical equation Ammonia (NH,) chemically reacts with oxygen gas (0,) to produce nitric oxide (NO) and water (H,0). What mass of nitric oxide is produced by the reaction of 2.65 g of oxygen gas? Be sure your answer has the correct number of significant digits. x10 Explanation Check 2020 McGraw-Hill Education. All Rights Re 10 MacBook Air II
A www-awn.aleks.com Significant Figures Counter Chapter 5. The Nervous System-Neurolo.. https://learn-us-east-1-prod-fleet01-xyth... ALEKS Sabina Lamgadhey - Learn O CHEMICAL REACTIONS Solving for a reactant using a chemical equation Ammonia (NH,) chemically reacts with oxygen gas (0,) to produce nitric oxide (NO) and water (H,0). What mass of nitric oxide is produced by the reaction of 2.65 g of oxygen gas? Be sure your answer has the correct number of significant digits. x10 Explanation Check 2020 McGraw-Hill Education. All Rights Re 10 MacBook Air II
Chapter11: Solving Equilibrium Problems For Complex Systems
Section: Chapter Questions
Problem 11.3QAP
Related questions
Question
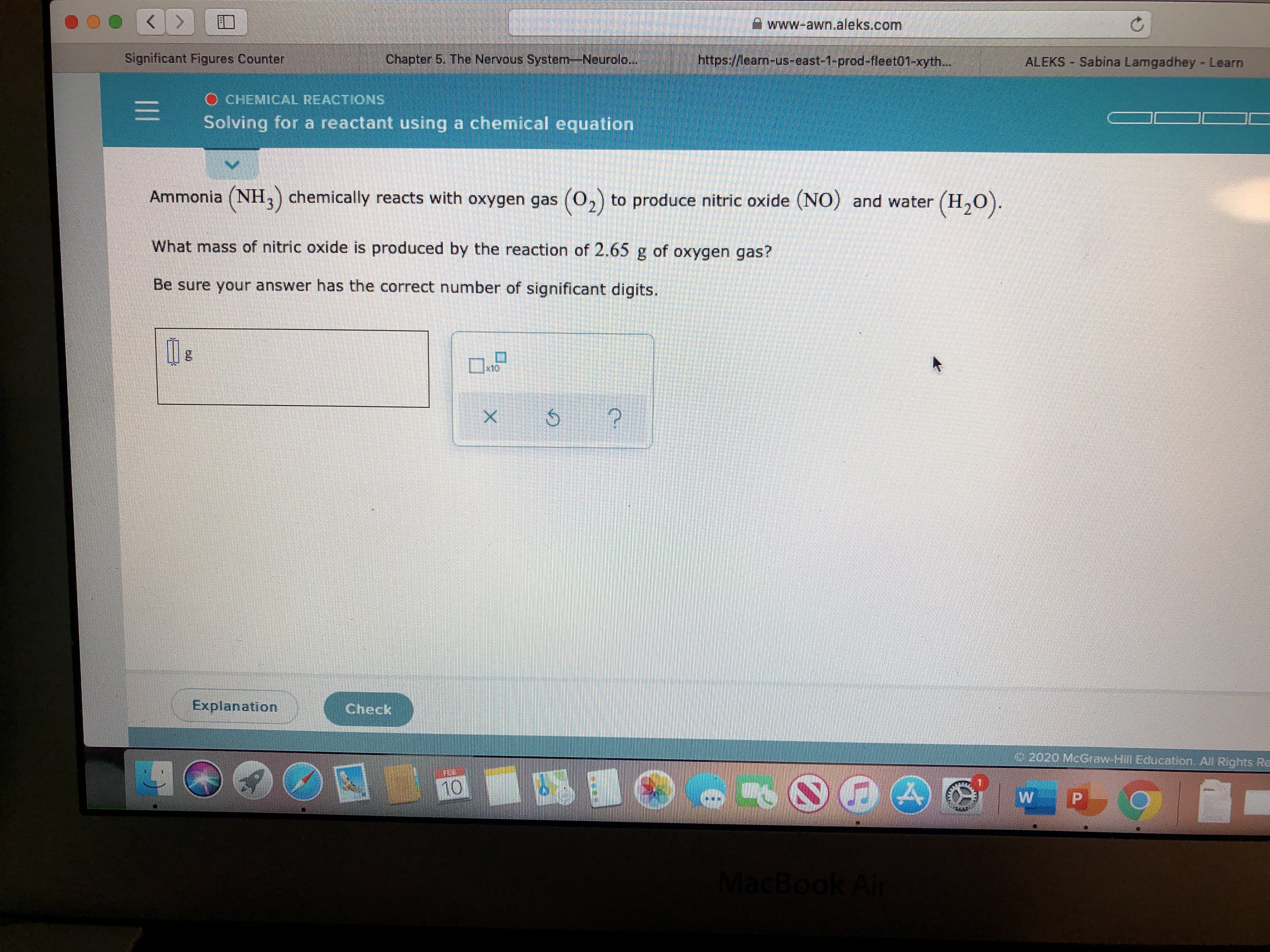
Transcribed Image Text:A www-awn.aleks.com
Significant Figures Counter
Chapter 5. The Nervous System-Neurolo..
https://learn-us-east-1-prod-fleet01-xyth...
ALEKS Sabina Lamgadhey - Learn
O CHEMICAL REACTIONS
Solving for a reactant using a chemical equation
Ammonia (NH,) chemically reacts with oxygen gas (0,) to produce nitric oxide (NO) and water (H,0).
What mass of nitric oxide is produced by the reaction of 2.65 g of oxygen gas?
Be sure your answer has the correct number of significant digits.
x10
Explanation
Check
2020 McGraw-Hill Education. All Rights Re
10
MacBook Air
II
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 4 steps with 4 images

Recommended textbooks for you

