To what decimal place should each answer be rounded? How many significant figures does the rounded answer have? 101 cm – 99.1 cm = 1.9 cm (unrounded) After rounding, the answer should be reported to the and thus has 3 significant figures ones place v 3 significant figures hundredths place 1 significant figure 0.5 atm + 9.8 atm = 10.3 atm (unrounded) tenths place 2 significant figures After rounding, the answer should be reported to the and thus has hundredths place ones place tenths place
To what decimal place should each answer be rounded? How many significant figures does the rounded answer have? 101 cm – 99.1 cm = 1.9 cm (unrounded) After rounding, the answer should be reported to the and thus has 3 significant figures ones place v 3 significant figures hundredths place 1 significant figure 0.5 atm + 9.8 atm = 10.3 atm (unrounded) tenths place 2 significant figures After rounding, the answer should be reported to the and thus has hundredths place ones place tenths place
Chemistry for Today: General, Organic, and Biochemistry
9th Edition
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Chapter6: The States Of Matter
Section: Chapter Questions
Problem 6.103E: What are the differentiating factors between potential and kinetic energy? a.Properties-physical or...
Related questions
Question
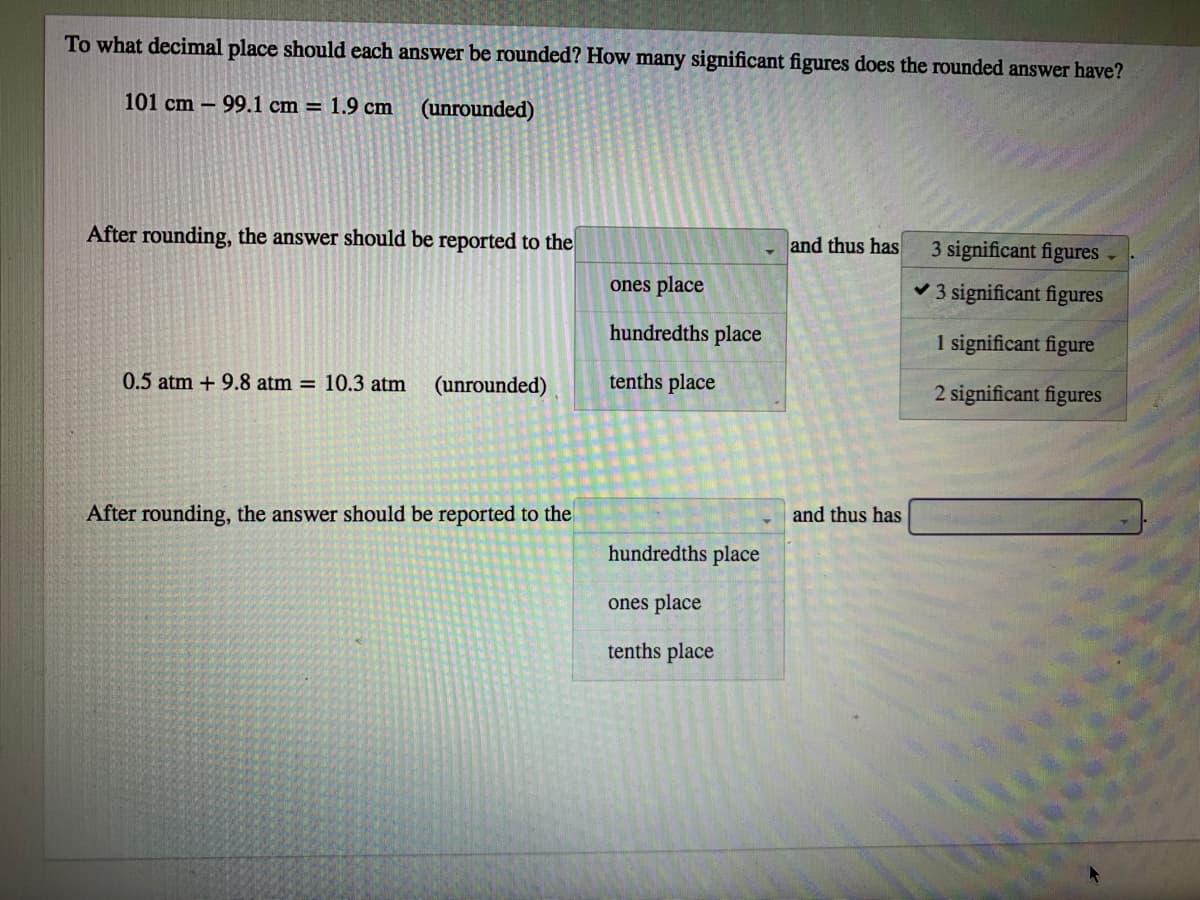
Transcribed Image Text:To what decimal place should each answer be rounded? How many significant figures does the rounded answer have?
101 cm – 99.1 cm = 1.9 cm
(unrounded)
After rounding, the answer should be reported to the
and thus has
3 significant figures
ones place
3 significant figures
hundredths place
1 significant figure
0.5 atm + 9.8 atm = 10.3 atm
(unrounded)
tenths place
2 significant figures
After rounding, the answer should be reported to the
and thus has
hundredths place
ones place
tenths place
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning