tra A Gaseous methane (CH) will react with gaseous oxygen (O,) to produce gaseous carbon dioxide (CO,) and gaseous water (H,O). Suppose 9.3 of methane g is mixed with 27.1 g of oxygen. Calculate the maximum mass of water that could be produced by the chemical reaction. Round your answer to 3 significant digits. X10 olo X S E Ar Check Explanation Terms of Use Privacy © 2019 McGraw-Hill Education. All Rights Resarved 11:15 PM 4x 17 12/3/2019 99+ Type here to search hp Bo end pg u home ins prt sc delete f10 f11 f12 f8 f9 144 f6 f4 f5 IOI esc + num backspace lock % 5 & 7 ! 3 4 6 7 2
tra A Gaseous methane (CH) will react with gaseous oxygen (O,) to produce gaseous carbon dioxide (CO,) and gaseous water (H,O). Suppose 9.3 of methane g is mixed with 27.1 g of oxygen. Calculate the maximum mass of water that could be produced by the chemical reaction. Round your answer to 3 significant digits. X10 olo X S E Ar Check Explanation Terms of Use Privacy © 2019 McGraw-Hill Education. All Rights Resarved 11:15 PM 4x 17 12/3/2019 99+ Type here to search hp Bo end pg u home ins prt sc delete f10 f11 f12 f8 f9 144 f6 f4 f5 IOI esc + num backspace lock % 5 & 7 ! 3 4 6 7 2
Chemistry: The Molecular Science
5th Edition
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:John W. Moore, Conrad L. Stanitski
Chapter10: Fuels, Organic Chemicals, And Polymers
Section10.2: U. S/ Energy Sources And Consumption
Problem 10.4E
Related questions
Question
100%
Transcribed Image Text:tra
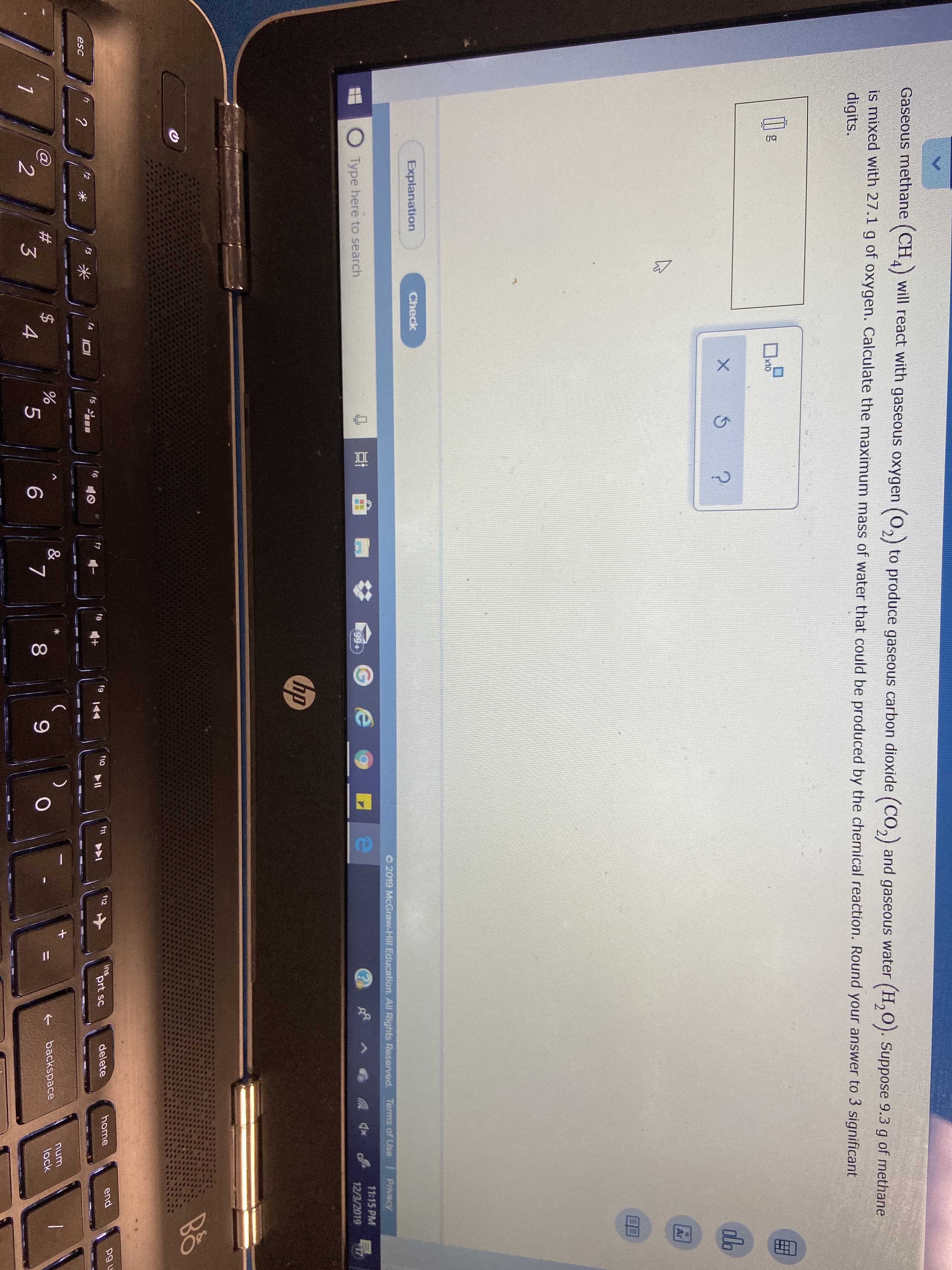
A
Gaseous methane (CH) will react with gaseous oxygen (O,) to produce gaseous carbon dioxide (CO,) and gaseous water (H,O). Suppose 9.3
of methane
g
is mixed with 27.1 g of oxygen. Calculate the maximum mass of water that could be produced by the chemical reaction. Round your answer to 3 significant
digits.
X10
olo
X
S
E
Ar
Check
Explanation
Terms of Use
Privacy
© 2019 McGraw-Hill Education. All Rights Resarved
11:15 PM
4x
17
12/3/2019
99+
Type here to search
hp
Bo
end
pg u
home
ins
prt sc
delete
f10
f11
f12
f8
f9
144
f6
f4
f5
IOI
esc
+
num
backspace
lock
%
5
&
7
!
3
4
6
7
2
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 2 images

Recommended textbooks for you

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning