True Value 124 g True Value 1.75 lb 1. 6. Trial # 1 120 g Trial # 1 2.75 lb Trial # 2 121 g Trial # 2 2.76 lb Trial # 3 120 g Trial # 3 2.78 lb True Value 1.15 L True Value 0.15 m 2. Trial # 1 0.95 L 7. Trial # 1 0.09 m Trial # 2 1.16 L Trial # 2 0.23 m Trial # 3 1.26 L. Trial # 3 0.36 m True Value 766 atm True Value 1.43 cm 3. 8. Trial # 1 765.9 atm Trial # 1 1.44 cm Trial # 2 765.8 atm Trial # 2 1.42 cm Trial # 3 766.4 atm Trial # 3 1. 40 cm True Value 10.1 N True Value 9.81 m/s Trial # 1 7 N 9. Trial # 1 14 m/s Trial # 2 3 N Trial # 2 8 m/s? Trial # 3 5 N Trial # 3 11 m/s True Value 15 psi True Value 36.5°C 5. 10. Trial # 1 20 psi Trial # 1 37.2 °C Tuinl n 11 noi Tuiol 4n 36 1 8r
True Value 124 g True Value 1.75 lb 1. 6. Trial # 1 120 g Trial # 1 2.75 lb Trial # 2 121 g Trial # 2 2.76 lb Trial # 3 120 g Trial # 3 2.78 lb True Value 1.15 L True Value 0.15 m 2. Trial # 1 0.95 L 7. Trial # 1 0.09 m Trial # 2 1.16 L Trial # 2 0.23 m Trial # 3 1.26 L. Trial # 3 0.36 m True Value 766 atm True Value 1.43 cm 3. 8. Trial # 1 765.9 atm Trial # 1 1.44 cm Trial # 2 765.8 atm Trial # 2 1.42 cm Trial # 3 766.4 atm Trial # 3 1. 40 cm True Value 10.1 N True Value 9.81 m/s Trial # 1 7 N 9. Trial # 1 14 m/s Trial # 2 3 N Trial # 2 8 m/s? Trial # 3 5 N Trial # 3 11 m/s True Value 15 psi True Value 36.5°C 5. 10. Trial # 1 20 psi Trial # 1 37.2 °C Tuinl n 11 noi Tuiol 4n 36 1 8r
Chapter8: Sampling, Standardization, And Calibration
Section: Chapter Questions
Problem 8.13QAP
Related questions
Question
Transcribed Image Text:V.
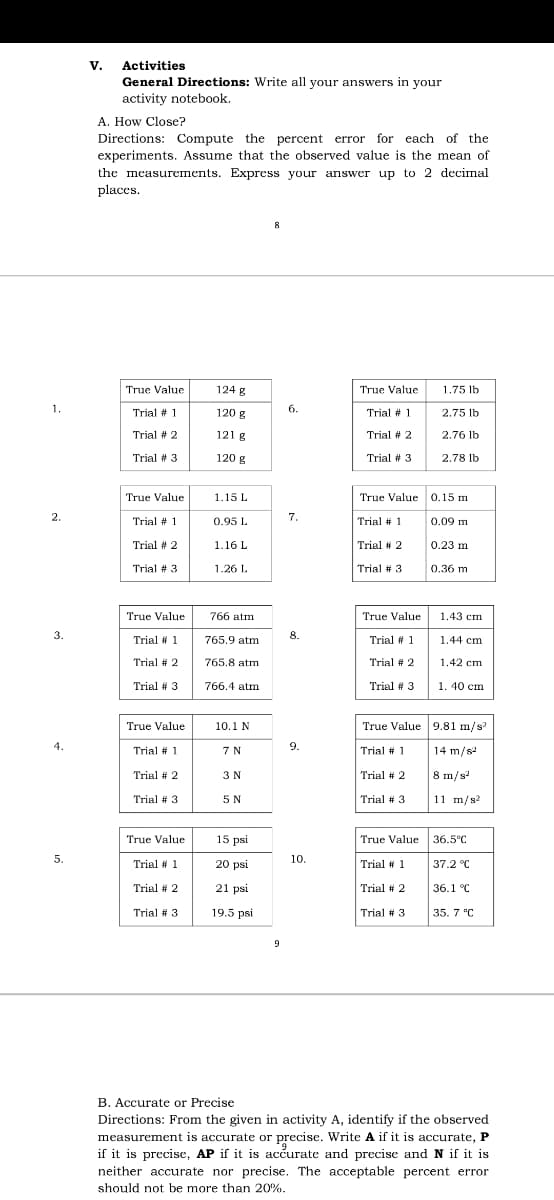
Activities
General Directions: Write all your answers in your
activity notebook.
A. How Close?
Directions: Compute the percent error for each of the
experiments. Assume that the observed value is the mean of
the measurements. Express your answer up to 2 decimal
places.
True Value
124 g
True Value
1.75 lb
1.
6.
Trial # 1
120 g
Trial # 1
2.75 lb
Trial # 2
121 g
Trial # 2
2.76 lb
Trial # 3
120 g
Trial # 3
2.78 lb
True Value
1.15 L
True Value 0.15 m
2.
7.
Trial # 1
0.95 L
Trial # 1
0.09 m
Trial # 2
1.16 L
Trial # 2
0.23 m
Trial # 3
1.26 L.
Trial # 3
0.36 m
True Value
766 atm
True Value
1.43 cm
3.
8.
Trial # 1
765.9 atm
Trial # 1
1.44 cm
Trial # 2
765.8 atm
Trial # 2
1.42 cm
Trial # 3
766.4 atm
Trial # 3
1. 40 cm
True Value
10.1 N
True Value 9.81 m/s
4.
9.
Trial # 1
7 N
Trial # 1
14 m/s
Trial # 2
3 N
Trial # 2
8 m/s
Trial # 3
5 N
Trial # 3
11 m/s?
True Value
15 psi
True Value 36.5°C
5.
10.
Trial # 1
20 psi
Trial # 1
37.2 °C
Trial # 2
21 psi
Trial # 2
36.1 °C
Trial # 3
19.5 psi
Trial # 3
35. 7 "C
9
B. Accurate or Precise
Directions: From the given in activity A, identify if the observed
measurement is accurate or precise. Write A if it is accurate, P
if it is precise, AP if it is acčurate and precise and N if it is
neither accurate nor precise. The acceptable percent error
should not be more than 20%.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Recommended textbooks for you



Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning



Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning