Tungsten is extracted from the mineral scheelite (CaWO4) by roasting it with natrite (Na2CO3) at a temperature of about 700 °C. This converts the CaWO4 to water soluble sodium tungstate (NazWO4). The solid product from the roaster (called "calcine") is transferred to a leacher. In the leacher chilled water is added to dissolve the NazWO4. The chilled water dissolves only the NazWO4 in the solid calcine. The scheelite that enters the roast is mixed with gypsum (CaSO4-2H2O). This stream is 85.2%w CaWO4 and 14.8%w CaSO4-2H2O. (%w means “percent-by-weight.") Four reactions take place during this process. In the roaster: 1: CaWO4 + Na2CO3 - NazWO4 + CaO + CO2 2: CaSO4•2H2O – CaSO4 + 2H2O In the leacher:
Tungsten is extracted from the mineral scheelite (CaWO4) by roasting it with natrite (Na2CO3) at a temperature of about 700 °C. This converts the CaWO4 to water soluble sodium tungstate (NazWO4). The solid product from the roaster (called "calcine") is transferred to a leacher. In the leacher chilled water is added to dissolve the NazWO4. The chilled water dissolves only the NazWO4 in the solid calcine. The scheelite that enters the roast is mixed with gypsum (CaSO4-2H2O). This stream is 85.2%w CaWO4 and 14.8%w CaSO4-2H2O. (%w means “percent-by-weight.") Four reactions take place during this process. In the roaster: 1: CaWO4 + Na2CO3 - NazWO4 + CaO + CO2 2: CaSO4•2H2O – CaSO4 + 2H2O In the leacher:
Principles of Modern Chemistry
8th Edition
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Chapter17: Electrochemistry
Section: Chapter Questions
Problem 80AP
Related questions
Question
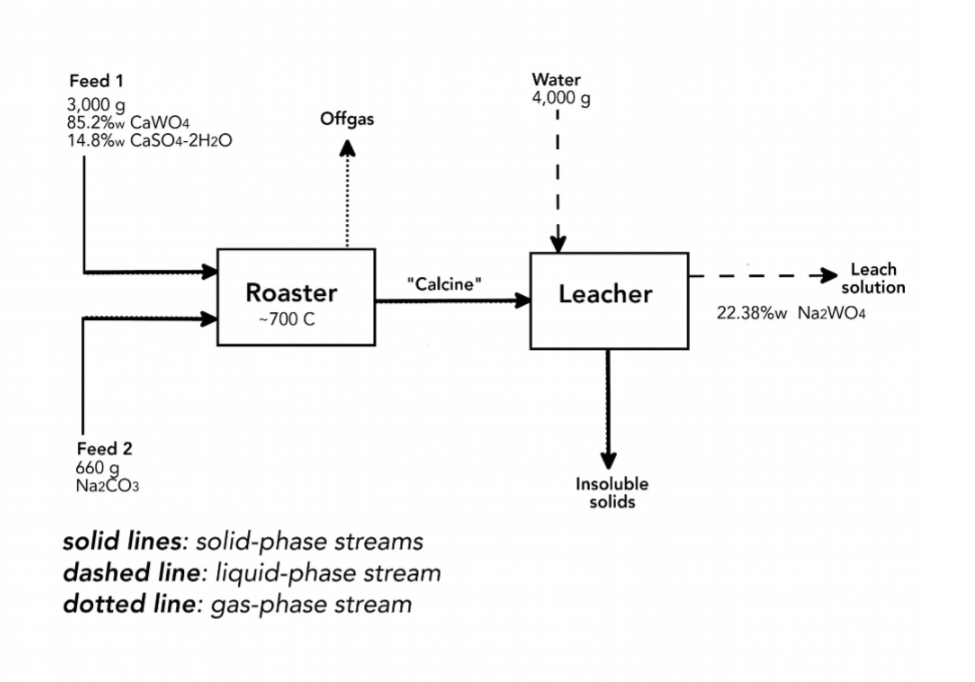
Transcribed Image Text:Feed 1
Water
4,000 g
3,000 g
85.2%w CaWO4
14.8%w CaSO4-2H2O
Offgas
Leach
solution
- - -
"Calcine"
Roaster
Leacher
~700 C
22.38%w Na2WO4
Feed 2
660 g
Na2ČO3
Insoluble
solids
solid lines: solid-phase streams
dashed line: liquid-phase stream
dotted line: gas-phase stream
Transcribed Image Text:Tungsten is extracted from the mineral scheelite (CaWO4) by roasting it with natrite (Na2CO3) at a
temperature of about 700 °C. This converts the CaWO4 to water soluble sodium tungstate (NazWO4).
The solid product from the roaster (called "calcine") is transferred to a leacher. In the leacher chilled
water is added to dissolve the NazWO4. The chilled water dissolves only the NazWO4 in the solid
calcine. The scheelite that enters the roast is mixed with gypsum (CaSO4-2H2O). This stream is
85.2%w CaWO4 and 14.8%w CaSO4-2H2O. (%w means “percent-by-weight.")
Four reactions take place during this process.
In the roaster:
1: CaWO4 + Na2CO3 - NazWO4 + CaO + CO2
2: CaSO4•2H2O - CaSO4 + 2H2O
In the leacher:
3: СаО + H2О —- Са(ОН)2
4: CaSO4 + 2H2O - CaSO4 2H2O
The first reaction does not go to completion, all the other reactions do go to completion.
For the conditions shown in the process diagram on the next page calculate the conversion of the
CaWO4 in the roaster.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 4 steps with 4 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning