%24 ALEKS - Jonnel Chippy - Learn https://www-awu.aleks.com/alekscgi/x/Isl.exe/1o_u-IgNslkr7j8P3jH-lis1SBdKFh4JYC7viZ6_juOtYisau7L4e3upirDI OATOMS, IONS AND MOLECULES Predicting the formula of binary ionic compounds Write the empirical formula of at least four binary ionic compounds that could be formed from the following ions: Zn, Al*, Br , Oo² 3+ I.. Explanation Check Te 2022 McGraw Hill LLC. AlI Rights Reserved. hulu N DE 58°F Mostly cloudy Home PrtScn F8 114 F3 F4 F5 F2 & 6 9- 2.
%24 ALEKS - Jonnel Chippy - Learn https://www-awu.aleks.com/alekscgi/x/Isl.exe/1o_u-IgNslkr7j8P3jH-lis1SBdKFh4JYC7viZ6_juOtYisau7L4e3upirDI OATOMS, IONS AND MOLECULES Predicting the formula of binary ionic compounds Write the empirical formula of at least four binary ionic compounds that could be formed from the following ions: Zn, Al*, Br , Oo² 3+ I.. Explanation Check Te 2022 McGraw Hill LLC. AlI Rights Reserved. hulu N DE 58°F Mostly cloudy Home PrtScn F8 114 F3 F4 F5 F2 & 6 9- 2.
World of Chemistry, 3rd edition
3rd Edition
ISBN:9781133109655
Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Chapter3: Chemical Foundations: Elements, Atoms, And Ions
Section: Chapter Questions
Problem 37A
Related questions
Question
Transcribed Image Text:%24
ALEKS - Jonnel Chippy - Learn
https://www-awu.aleks.com/alekscgi/x/Isl.exe/1o_u-IgNslkr7j8P3jH-lis1SBdKFh4JYC7viZ6_juOtYisau7L4e3upirDI
OATOMS, IONS AND MOLECULES
Predicting the formula of binary ionic compounds
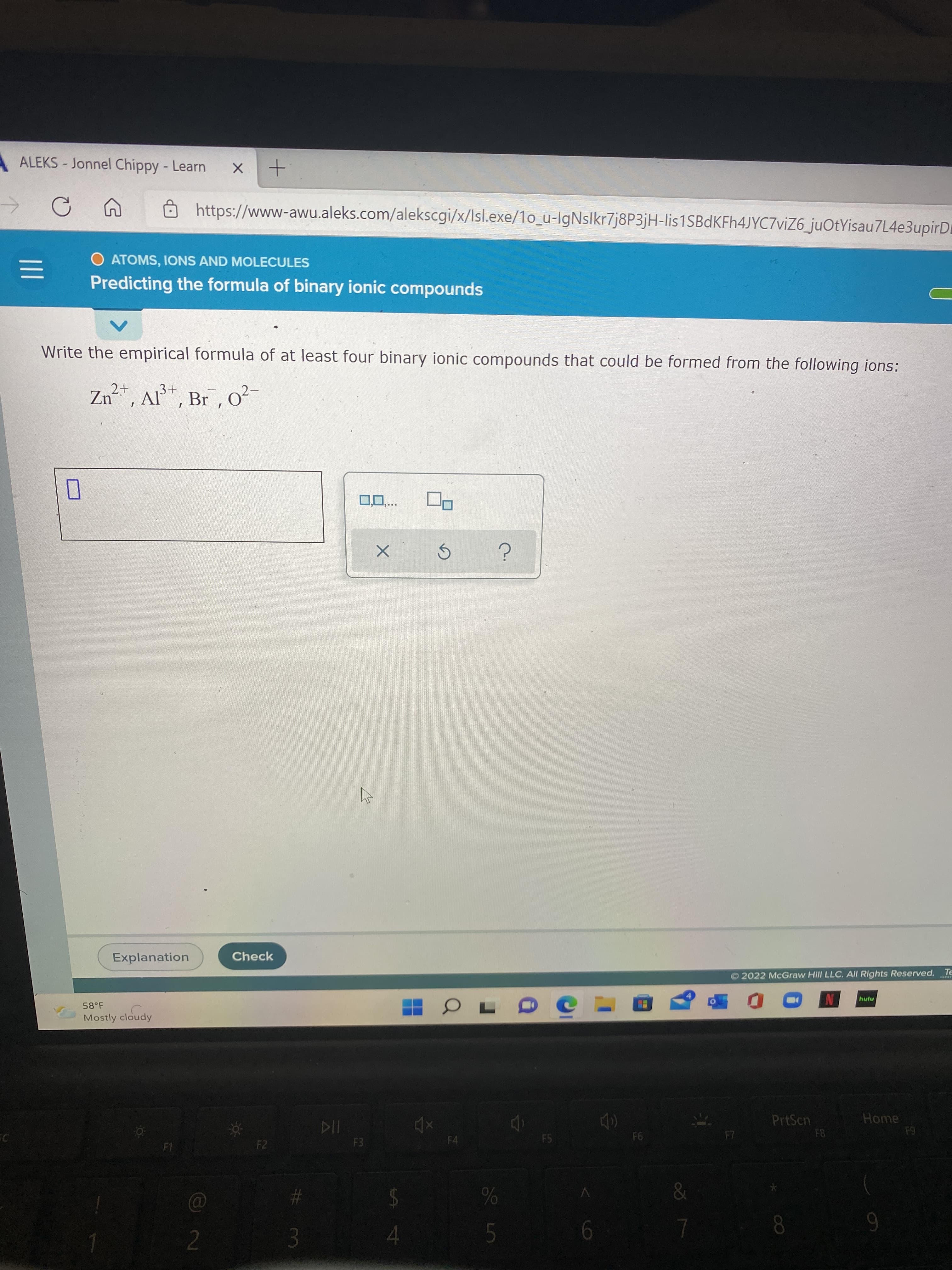
Write the empirical formula of at least four binary ionic compounds that could be formed from the following ions:
Zn, Al*, Br , Oo²
3+
I..
Explanation
Check
Te
2022 McGraw Hill LLC. AlI Rights Reserved.
hulu
N DE
58°F
Mostly cloudy
Home
PrtScn
F8
114
F3
F4
F5
F2
&
6
9-
2.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning