Two constant-volume gas thermometers are assembled, one with nitrogen and the other with hydrogen. Both contain enough gas so that p3 = 110 kPa. (a) What is the difference between the pressures in the two thermometers if both bulbs are in boiling water? (Hint: See the figure.) (b) Which gas is at higher pressure, nitrogen or hydrogen? 373.50 373.40 373.125 K Ng 373.30 373.20 H2 373.10 Не 20 40 60 80 100 120 Þ3 (kPa) Indications of the constant-volume gas thermometer for a boiling point of water depending on pressure at the triple point of water for various gases (a) Number Units (b) Use correct number of significant digits; the tolerance is +/-2% Temperature (K)
Two constant-volume gas thermometers are assembled, one with nitrogen and the other with hydrogen. Both contain enough gas so that p3 = 110 kPa. (a) What is the difference between the pressures in the two thermometers if both bulbs are in boiling water? (Hint: See the figure.) (b) Which gas is at higher pressure, nitrogen or hydrogen? 373.50 373.40 373.125 K Ng 373.30 373.20 H2 373.10 Не 20 40 60 80 100 120 Þ3 (kPa) Indications of the constant-volume gas thermometer for a boiling point of water depending on pressure at the triple point of water for various gases (a) Number Units (b) Use correct number of significant digits; the tolerance is +/-2% Temperature (K)
College Physics
10th Edition
ISBN:9781285737027
Author:Raymond A. Serway, Chris Vuille
Publisher:Raymond A. Serway, Chris Vuille
Chapter10: Thermal Physics
Section: Chapter Questions
Problem 7WUE: One way to cool a gas is to let it expand. When a certain gas under a pressure of 5.00 106 Ha at...
Related questions
Question
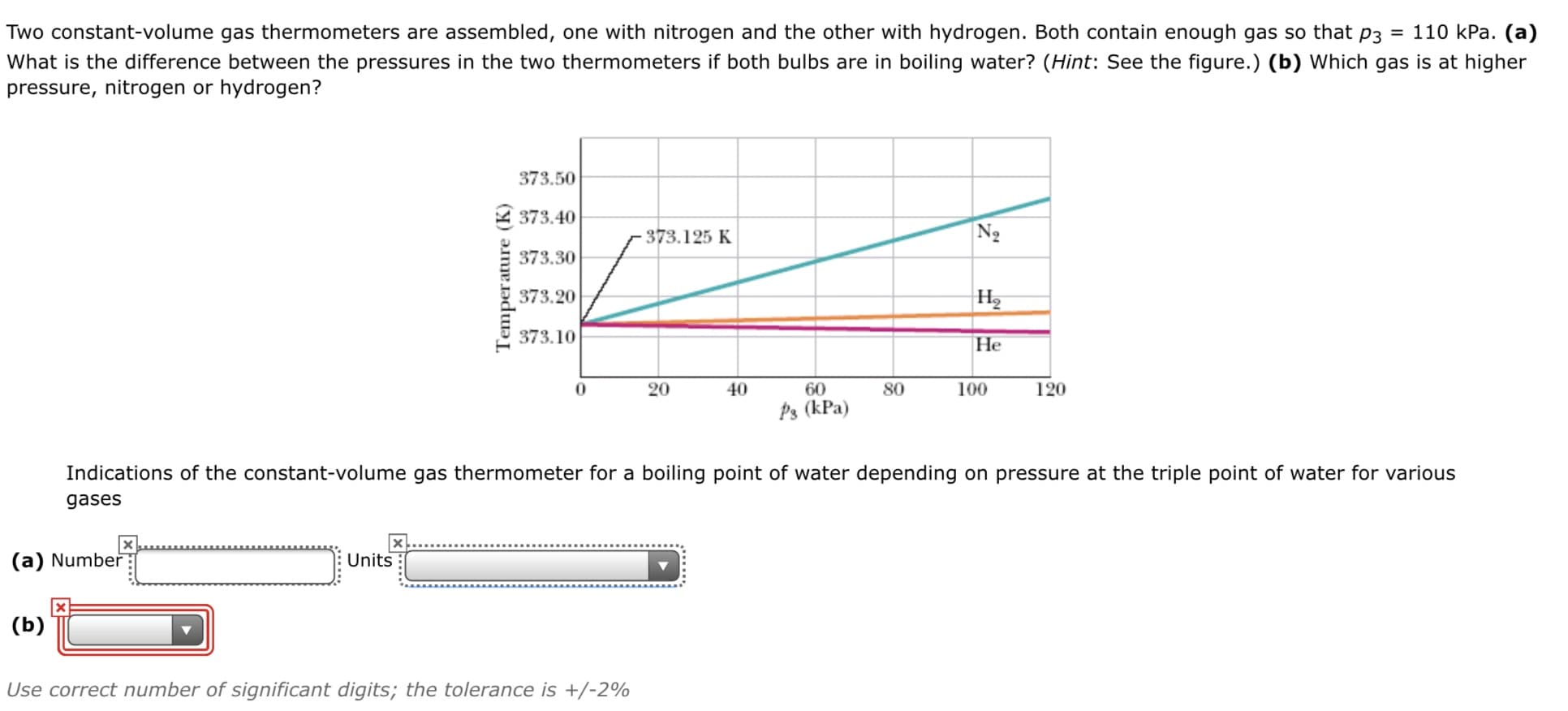
Transcribed Image Text:Two constant-volume gas thermometers are assembled, one with nitrogen and the other with hydrogen. Both contain enough gas so that p3 = 110 kPa. (a)
What is the difference between the pressures in the two thermometers if both bulbs are in boiling water? (Hint: See the figure.) (b) Which gas is at higher
pressure, nitrogen or hydrogen?
373.50
373.40
373.125 K
Ng
373.30
373.20
H2
373.10
Не
20
40
60
80
100
120
Þ3 (kPa)
Indications of the constant-volume gas thermometer for a boiling point of water depending on pressure at the triple point of water for various
gases
(a) Number
Units
(b)
Use correct number of significant digits; the tolerance is +/-2%
Temperature (K)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 5 steps with 5 images

Recommended textbooks for you

College Physics
Physics
ISBN:
9781285737027
Author:
Raymond A. Serway, Chris Vuille
Publisher:
Cengage Learning

Physics for Scientists and Engineers, Technology …
Physics
ISBN:
9781305116399
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning

Principles of Physics: A Calculus-Based Text
Physics
ISBN:
9781133104261
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning

College Physics
Physics
ISBN:
9781285737027
Author:
Raymond A. Serway, Chris Vuille
Publisher:
Cengage Learning

Physics for Scientists and Engineers, Technology …
Physics
ISBN:
9781305116399
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning

Principles of Physics: A Calculus-Based Text
Physics
ISBN:
9781133104261
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning

Physics for Scientists and Engineers: Foundations…
Physics
ISBN:
9781133939146
Author:
Katz, Debora M.
Publisher:
Cengage Learning

An Introduction to Physical Science
Physics
ISBN:
9781305079137
Author:
James Shipman, Jerry D. Wilson, Charles A. Higgins, Omar Torres
Publisher:
Cengage Learning

College Physics
Physics
ISBN:
9781305952300
Author:
Raymond A. Serway, Chris Vuille
Publisher:
Cengage Learning